当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intramolecular Nucleophilic Substitution of ω-Haloalkylphosphine Derivatives
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acs.joc.7b01767 Paweł Woźnicki 1 , Ewelina Korzeniowska 1 , Marek Stankevič 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acs.joc.7b01767 Paweł Woźnicki 1 , Ewelina Korzeniowska 1 , Marek Stankevič 1
Affiliation
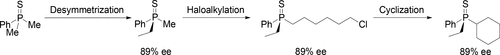
|
ω-Haloalkylphosphine derivatives undergo the intramolecular nucleophilic substitution reaction upon treatment with a strong base, yielding either cycloalkylphosphine derivatives or heterocyclic phosphine derivatives. The selectivity of the cyclization of (ω-haloalkyl)alkylarylphosphine derivatives depends strongly on the distance between the electrophilic and nucleophilic carbon atoms and the structure of the phosphorus moiety. The desymmetrization of dimethylphenylphosphine sulfide followed by haloalkylation and cyclization led to the enantiomerically enriched tertiary phosphine sulfide, possessing a cyclohexyl fragment at the phosphorus.
中文翻译:

ω-卤代烷基膦衍生物的分子内亲核取代
用强碱处理后,ω-卤代烷基膦衍生物经历分子内亲核取代反应,产生环烷基膦衍生物或杂环膦衍生物。(ω-卤代烷基)烷基芳基膦衍生物的环化的选择性很大程度上取决于亲电和亲核碳原子之间的距离以及磷部分的结构。二甲基苯基膦硫化物的脱对称化,然后是卤代烷基化和环化作用,导致对映体富集的叔膦硫化物,在磷处具有一个环己基片段。
更新日期:2017-09-13
中文翻译:

ω-卤代烷基膦衍生物的分子内亲核取代
用强碱处理后,ω-卤代烷基膦衍生物经历分子内亲核取代反应,产生环烷基膦衍生物或杂环膦衍生物。(ω-卤代烷基)烷基芳基膦衍生物的环化的选择性很大程度上取决于亲电和亲核碳原子之间的距离以及磷部分的结构。二甲基苯基膦硫化物的脱对称化,然后是卤代烷基化和环化作用,导致对映体富集的叔膦硫化物,在磷处具有一个环己基片段。











































 京公网安备 11010802027423号
京公网安备 11010802027423号