Advanced Drug Delivery Reviews ( IF 15.2 ) Pub Date : 2017-09-13 , DOI: 10.1016/j.addr.2017.09.004 Ulrich Martin
|
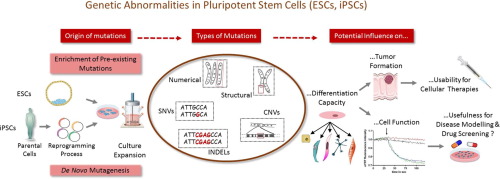
Inherited and acquired genomic abnormalities are known to cause genetic diseases and contribute to cancer formation. Recent studies demonstrated a substantial mutational load in mouse and human embryonic and induced pluripotent stem cells (ESCs and iPSCs). Single nucleotide variants, copy number variations, and larger chromosomal abnormalities may influence the differentiation capacity of pluripotent stem cells and the functionality of their derivatives in disease modeling and drug screening, and are considered a serious risk for cellular therapies based on ESC or iPSC derivatives. This review discusses the types and origins of different genetic abnormalities in pluripotent stem cells, methods for their detection, and the mechanisms of development and enrichment during reprogramming and culture expansion.
中文翻译:

程序化干细胞产品的基因组稳定性
已知遗传和获得性基因组异常会导致遗传疾病并导致癌症形成。最近的研究表明,在小鼠和人类胚胎及诱导的多能干细胞(ESC和iPSC)中存在大量的突变负荷。单核苷酸变异,拷贝数变异和较大的染色体异常可能会影响多能干细胞的分化能力及其衍生物在疾病建模和药物筛选中的功能,并被认为是基于ESC或iPSC衍生物进行细胞疗法的严重风险。这篇综述讨论了多能干细胞中不同遗传异常的类型和起源,它们的检测方法以及重编程和培养扩增过程中发育和富集的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号