Tetrahedron ( IF 2.1 ) Pub Date : 2017-09-13 , DOI: 10.1016/j.tet.2017.09.014 Aleksandra Minić , Dragana Stevanović , Mirjana Vukićević , Goran A. Bogdanović , Matthias D'hooghe , Niko S. Radulović , Rastko D. Vukićević
|
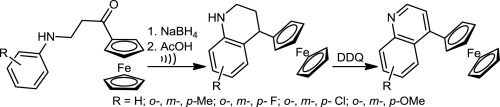
A series of novel 4-ferrocenyl-1,2,3,4-tetrahydroquinolines were synthesized in high-to-excellent yields (up to 99%) starting from the corresponding ferrocenoylethyl aryl amines. These Mannich bases were reduced (NaBH4) to the corresponding 3-(arylamino)-1-ferrocenylpropan-1-ols and submitted to an intramolecular cyclization prompted by acetic acid, proceeding via the corresponding α-ferrocenyl carbenium ion intermediates. Subsequently, the obtained tetrahydroquinolines were smoothly oxidized (aromatized) by means of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) to provide the corresponding 4-ferrocenylquinolines (up to 93%).
中文翻译:

以α-二茂铁基碳鎓离子为关键中间体,合成新型4-二茂铁基-1,2,3,4-四氢喹啉和4-二茂铁喹啉
从相应的二茂铁酰基乙基芳基胺开始,以高至优异的产率(高达99%)合成了一系列新颖的4-二茂铁基-1,2,3,4-四氢喹啉。将这些曼尼希碱还原(NaBH 4)为相应的3-(芳基氨基)-1-二茂铁基丙烷-1-醇,并通过乙酸进行分子内环化,然后通过相应的α-二茂铁基碳鎓离子中间体进行。随后,将所获得的四氢喹啉通过2,3-二氯-5,6-二氰基-1,4-苯醌(DDQ)平滑地氧化(芳香化),以提供相应的4-二茂铁基喹啉(至多93%)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号