当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Half-Sandwich Ruthenium Catalyst Bearing an Enantiopure Primary Amine Tethered to an N-Heterocyclic Carbene for Ketone Hydrogenation
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1021/acscatal.7b02346 Kai Y. Wan 1 , Molly M. H. Sung 1 , Alan J. Lough 1 , Robert H. Morris 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1021/acscatal.7b02346 Kai Y. Wan 1 , Molly M. H. Sung 1 , Alan J. Lough 1 , Robert H. Morris 1
Affiliation
|
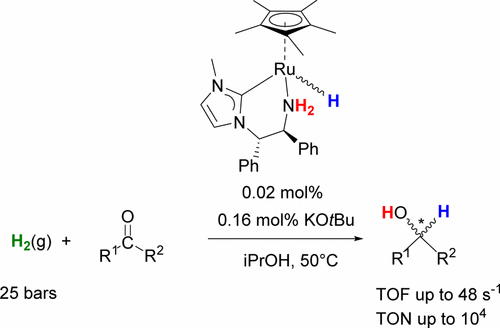
By using a copper transmetalation reagent [Cu(Kaibene)2]I, the NHC ligand (S,S)-MeNC3H2NCHPhCHPhNH2 “Kaibene” was transferred to ruthenium to make a precatalyst [RuCp*(Kaibene)(MeCN)](PF6) (Cp* = 1,2,3,4,5-pentamethylcyclopentadienyl), 7, in high yield as a mixture of two diastereomers. Under relatively mild conditions (0.02 mol % Ru, 0.16 mol % KOtBu, iPrOH, 50 °C, 25 bar of H2), this compound catalyzes the hydrogenation of aryl ketones and one alkyl ketone effectively with excellent activity and productivity (TOF up to 48 s–1, TON up to 104). At higher hydrogenation pressure (46 bar), the catalytic hydrogenation of N-phenyl-benzylimine to the corresponding amine is efficiently achieved. The hydrogenation of prochiral ketones resulted in low ee (35% for 4-chloroacetophenone). NMR spectroscopy was used to observe diastereomeric hydrides RuCp*(Kaibene)(H) 13-R/S that were generated by reaction of 7 with H2 and base in THF-d8. Complementary DFT studies suggest that either the heterolytic splitting of dihydrogen to form 13-R/S or the hydride transfer to the substrate can be rate-determining depending on the substrate. Experimental and computational results support mechanisms that involve the heterolytic splitting of dihydrogen to the nitrogen of the amide-ligated form of Kaibene in THF or the heterolytic splitting to an outer-sphere alkoxide derived from the product alcohol or 2-PrOH solvent. An unusual feature is the rapid drop in ee of the product alcohol from as high as 60% (R) to 0% in some cases; this might be due to racemization of the Kaibene ligand in THF caused by the strong base or competitive inhibition of one diastereomer of the catalyst by reaction with the product (R)-alcohol.
中文翻译:

带有对映纯伯胺的半三明治钌催化剂,该胺系留在N-杂环碳烯上进行酮加氢
通过使用铜金属转移试剂[Cu(Kaibene)2 ] I,将NHC配体(S,S)-MeNC 3 H 2 NCHPhCHPhNH 2 “ Kaibene”转移到钌上,制成前催化剂[RuCp *(Kaibene)(MeCN) ]] [PF 6 ](Cp * = 1,2,3,4,5-五甲基环戊二烯基),7,两种非对映异构体的混合物,收率很高。在相对温和的条件下(0.02 mol%Ru,0.16 mol%KOtBu,iPrOH,50°C,25 bar H 2),该化合物可有效催化芳基酮和一种烷基酮的氢化反应,并具有出色的活性和生产率(TOF最高可达48 s –1,TON至10 4)。在较高的氢化压力(46 bar)下,可以有效地实现N-苯基-苄基亚胺的催化氢化为相应的胺。前手性酮的氢化导致较低的ee(4-氯苯乙酮为35%)。NMR光谱用于观察非对映氢化物RuCp *(Kaibene)(H)13- R / S,其由7与H 2和碱在THF- d 8中反应生成。DFT的补充研究表明,要么氢的异源裂解形成13- R / S或氢化物转移到基材上的速率可以取决于基材。实验和计算结果支持了以下机制,其中涉及将二氢杂解拆分为酰胺键形式的Kaibene在THF中的氮或杂解拆分为衍生自产物醇或2-PrOH溶剂的外球体醇盐。一个不寻常的特征是产品酒精的ee迅速从高达60%(R)下降到0%。这可能是由于Kaebene配体在THF中的外消旋作用所致,该起因是由于与产物(R)醇反应而对催化剂的一种非对映异构体具有强碱或竞争性抑制作用。
更新日期:2017-09-12
中文翻译:

带有对映纯伯胺的半三明治钌催化剂,该胺系留在N-杂环碳烯上进行酮加氢
通过使用铜金属转移试剂[Cu(Kaibene)2 ] I,将NHC配体(S,S)-MeNC 3 H 2 NCHPhCHPhNH 2 “ Kaibene”转移到钌上,制成前催化剂[RuCp *(Kaibene)(MeCN) ]] [PF 6 ](Cp * = 1,2,3,4,5-五甲基环戊二烯基),7,两种非对映异构体的混合物,收率很高。在相对温和的条件下(0.02 mol%Ru,0.16 mol%KOtBu,iPrOH,50°C,25 bar H 2),该化合物可有效催化芳基酮和一种烷基酮的氢化反应,并具有出色的活性和生产率(TOF最高可达48 s –1,TON至10 4)。在较高的氢化压力(46 bar)下,可以有效地实现N-苯基-苄基亚胺的催化氢化为相应的胺。前手性酮的氢化导致较低的ee(4-氯苯乙酮为35%)。NMR光谱用于观察非对映氢化物RuCp *(Kaibene)(H)13- R / S,其由7与H 2和碱在THF- d 8中反应生成。DFT的补充研究表明,要么氢的异源裂解形成13- R / S或氢化物转移到基材上的速率可以取决于基材。实验和计算结果支持了以下机制,其中涉及将二氢杂解拆分为酰胺键形式的Kaibene在THF中的氮或杂解拆分为衍生自产物醇或2-PrOH溶剂的外球体醇盐。一个不寻常的特征是产品酒精的ee迅速从高达60%(R)下降到0%。这可能是由于Kaebene配体在THF中的外消旋作用所致,该起因是由于与产物(R)醇反应而对催化剂的一种非对映异构体具有强碱或竞争性抑制作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号