当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental and Computational Investigation of Salophen–Zn Gas Phase Complexes with Cations: A Source of Possible Interference in Anionic Recognition
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1021/acs.jpca.7b05825 Alessandra Ciavardini 1 , Simonetta Fornarini 1 , Antonella Dalla Cort 2 , Susanna Piccirillo 3 , Debora Scuderi 4 , Enrico Bodo 5
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1021/acs.jpca.7b05825 Alessandra Ciavardini 1 , Simonetta Fornarini 1 , Antonella Dalla Cort 2 , Susanna Piccirillo 3 , Debora Scuderi 4 , Enrico Bodo 5
Affiliation
|
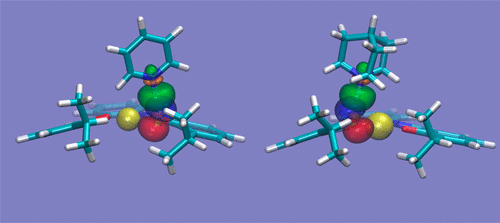
We explore the possibility that protonated molecular ions might be an unexpected source of interference in the recognition process of anions and neutral species by Zn–salophen receptors. Zn–salophen complexes are known to bind anions and neutral molecules in solution. We present here evidence (from computational work and IRMPD spectroscopy) that these complexes can also be the binding site for protonated pyridine or quinuclidine. The resulting binding pattern does not involve the Zn ion, but one of the oxygen atoms directly attached to it. The resulting complex therefore turns out to have a positive charge adjacent to the Zn–salophen binding site. This finding seems to point to the existence of an interfering factor in the quantification of the experimental data about the association constant.
中文翻译:

具有阳离子的Salophen-Zn气相配合物的实验和计算研究:阴离子识别中可能的干扰源
我们探讨了质子化的分子离子可能是Zn-salophen受体识别阴离子和中性物质过程中意料之外的干扰源的可能性。众所周知,锌-salophen配合物会结合阴离子和溶液中的中性分子。我们在这里提供证据(来自计算工作和IRMPD光谱),这些复合物也可以是质子化吡啶或奎宁环的结合位点。最终的结合模式不涉及Zn离子,而是一个直接与之相连的氧原子。因此,所形成的复合物在Zn-salophen结合位点附近具有正电荷。这一发现似乎表明在有关缔合常数的实验数据的量化中存在干扰因素。
更新日期:2017-09-12
中文翻译:

具有阳离子的Salophen-Zn气相配合物的实验和计算研究:阴离子识别中可能的干扰源
我们探讨了质子化的分子离子可能是Zn-salophen受体识别阴离子和中性物质过程中意料之外的干扰源的可能性。众所周知,锌-salophen配合物会结合阴离子和溶液中的中性分子。我们在这里提供证据(来自计算工作和IRMPD光谱),这些复合物也可以是质子化吡啶或奎宁环的结合位点。最终的结合模式不涉及Zn离子,而是一个直接与之相连的氧原子。因此,所形成的复合物在Zn-salophen结合位点附近具有正电荷。这一发现似乎表明在有关缔合常数的实验数据的量化中存在干扰因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号