Dyes and Pigments ( IF 4.1 ) Pub Date : 2017-09-12 , DOI: 10.1016/j.dyepig.2017.09.023 Rafaela Conceição , Graham Hungerford , Susana P.G. Costa , M. Sameiro T. Gonçalves
|
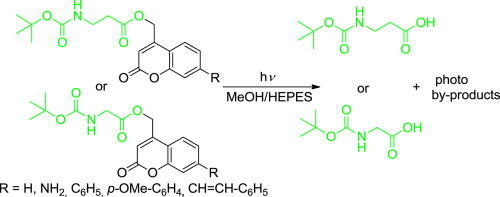
New ester cages bearing the coumarin (2H-benzopyran-2-one) skeleton with extended π-systems as phototriggers, for glycine and β-alanine, as models of carboxylic acid bifunctional molecules with biological relevance, were evaluated under photolysis conditions at 254, 300, 350 and 419 nm of irradiation in a RPR-100 photochemical reactor. The processes were followed by HPLC-UV detection and 1H NMR with collection of kinetic data. The results showed a correlation between the photolysis efficiency and the increasing extension of the conjugation for both glycine and β-alanine, showing that the 7-aminocoumarin afforded the best results at all wavelengths tested.
From a study of the time-resolved fluorescence behaviour, these compounds were also found to exhibit more complex fluorescence decay kinetics. This was attributed to the presence of conjugated and non-conjugated coumarin species.
中文翻译:

从稠合吡喃共轭物中光解生物活性羧酸
在254的光解条件下,评估了带有香豆素(2 H-苯并吡喃-2-酮)骨架并具有扩展的π系统作为光触发的新酯笼,用于光触发的甘氨酸和β-丙氨酸作为具有生物相关性的羧酸双功能分子模型在RPR-100光化学反应器中照射300、350和419 nm。该过程之后进行HPLC-UV检测和1 H NMR,并收集动力学数据。结果表明,甘氨酸和β-丙氨酸的光解效率与共轭作用的增加扩展之间存在相关性,表明7-氨基香豆素在所有测试波长下均能提供最佳结果。
通过对时间分辨荧光行为的研究,还发现这些化合物表现出更复杂的荧光衰减动力学。这归因于共轭和非共轭香豆素种类的存在。









































 京公网安备 11010802027423号
京公网安备 11010802027423号