Molecular Catalysis ( IF 3.9 ) Pub Date : 2017-09-12 , DOI: 10.1016/j.mcat.2017.08.011 Xiaowa Nie , Xiaojing Ji , Yonggang Chen , Xinwen Guo , Chunshan Song
|
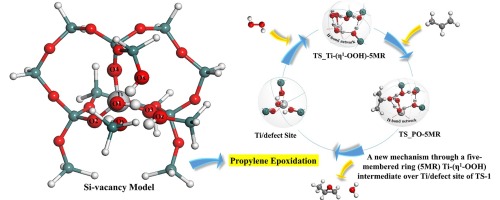
Density functional theory (DFT) calculations were performed to investigate propylene epoxidation mechanisms with H2O2 over various TS-1 models in terms of active site formation, intermediate identification, and oxygen transfer pathway. Over tripodal sites of the hydrolyzed model, a bidentate Ti-OOH intermediate assembled in the 3-membered ring (3MR) configuration (Ti-(η2-OOH)-3MR) was identified through the stepwise mechanism for propylene epoxidation while a monodentate Ti-H2O2 intermediate stabilized in the 5MR configuration (Ti-(η1-H2O2)-5MR) was obtained via the concerted mechanism. Both stepwise and concerted paths were found to be possible over the hydrolyzed models. On Ti/defect site of the Si-vacancy model, a new mechanism through a 5MR Ti-(η1-OOH) intermediate was demonstrated to be energetically the most favorable candidate to represent the propylene epoxidation chemistry in H2O2/TS-1. The barriers (5.1 and 10.4 kcal/mol) for H2O2 dissociation and epoxidation are both relatively lower as compared to literature, which can be attributed to the steric advantage of the Si-vacancy model as well as the H-bond networks formed with surrounding silanol groups. The effect of co-produced H2O on propylene epoxidation kinetics was also examined and the calculation results showed that H2O coordination to the Ti center facilitates the formation of 5MR Ti-(η1-OOH) intermediate and dramatically lowers the barriers for both H2O2 dissociation and epoxidation over the Ti/defect site of TS-1.
中文翻译:

TS-1上H 2 O 2与丙烯环氧化的机理研究:活性位形成,中间体鉴定和氧转移途径
进行密度泛函理论(DFT)计算以研究在各种TS-1模型上H 2 O 2引起的丙烯环氧化机理,这些机理涉及活性位点形成,中间识别和氧转移途径。水解模型,双齿的Ti-OOH中间体在3元环组装超过三角架位点(3MR)配置(性Ti-(η 2 -OOH)-3MR)通过逐步机制丙烯环氧化被认定而单齿的Ti -H 2 Ó 2在5MR配置(性Ti-(η稳定中间体1 -H 2 Ò 2)-5MR)是通过协同机制获得的。发现在水解模型上,步进路径和协同路径都是可行的。对Ti /硅空位模型的缺损部位,通过5MR性Ti-的新机制(η 1 -OOH)被证明是能量上最有利的候选来表示丙烯环氧化化学中间体H中2 ö 2 / TS- 1。与文献相比,H 2 O 2离解和环氧化的势垒(5.1和10.4 kcal / mol)都相对较低,这可以归因于Si空位模型的空间优势以及形成的H键网络与周围的硅烷醇基团。共同产生的H 2的作用上丙烯环氧化动力学O另检查和的计算结果表明使得h 2 ö协调与Ti中心便于5MR性Ti-的形成(η 1 -OOH)中间,并显着降低了均为H的障碍2 Ò 2解离和环氧化在TS-1的Ti /缺陷部位上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号