当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium-Catalyzed Synthesis of Multiaryl-substituted Naphthols via a Removable Directing Group
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-09-12 07:05:53 , DOI: 10.1002/adsc.201700726 Lianhui Wang 1 , Yunliang Yu 1 , Mengqi Yang 1 , Changsheng Kuai 1 , Dingding Cai 1 , Jinfeng Yu 1 , Xiuling Cui 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-09-12 07:05:53 , DOI: 10.1002/adsc.201700726 Lianhui Wang 1 , Yunliang Yu 1 , Mengqi Yang 1 , Changsheng Kuai 1 , Dingding Cai 1 , Jinfeng Yu 1 , Xiuling Cui 1
Affiliation
|
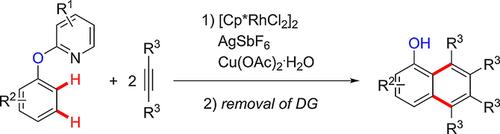
Arene homologation employing internal alkynes as coupling partners and 2-pyridyloxyl as directing group through dual C−H bond functionalization was accomplished using (pentamethylcyclopentadienyl)-rhodium(III) chloride dimer as a pre-catalyst. This protocol proved tolerant of synthetically valuable functional groups, and provided an expeditious access to highly congested naphthalene derivatives in moderate to good yields. Furthermore, the pyridyl moiety could be removed to furnish the versatile (OH)-free naphthols.
中文翻译:

铑通过可移动的导向基团催化多芳基取代的萘酚的合成
使用内部戊炔作为偶合配偶体和2-吡啶氧基作为通过双CH键官能化的指导基团的芳烃同系物是使用(五甲基环戊二烯基)氯化铑(III)二聚体作为前催化剂完成的。该方案证明对合成有价值的官能团具有耐受性,并提供了以中等到良好的产率快速获得高度拥挤的萘衍生物的途径。此外,可以去除吡啶基部分以提供通用的无(OH)的萘酚。
更新日期:2017-09-12
中文翻译:

铑通过可移动的导向基团催化多芳基取代的萘酚的合成
使用内部戊炔作为偶合配偶体和2-吡啶氧基作为通过双CH键官能化的指导基团的芳烃同系物是使用(五甲基环戊二烯基)氯化铑(III)二聚体作为前催化剂完成的。该方案证明对合成有价值的官能团具有耐受性,并提供了以中等到良好的产率快速获得高度拥挤的萘衍生物的途径。此外,可以去除吡啶基部分以提供通用的无(OH)的萘酚。











































 京公网安备 11010802027423号
京公网安备 11010802027423号