当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A redox-switchable ring-closing metathesis catalyst
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2017-06-13 00:00:00 , DOI: 10.1039/c7qi00018a Dominika N. Lastovickova 1, 2, 3, 4, 5 , Aaron J. Teator 1, 2, 3, 4, 5 , Huiling Shao 1, 5, 6, 7 , Peng Liu 1, 5, 6, 7 , Christopher W. Bielawski 1, 8, 9, 10, 11
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2017-06-13 00:00:00 , DOI: 10.1039/c7qi00018a Dominika N. Lastovickova 1, 2, 3, 4, 5 , Aaron J. Teator 1, 2, 3, 4, 5 , Huiling Shao 1, 5, 6, 7 , Peng Liu 1, 5, 6, 7 , Christopher W. Bielawski 1, 8, 9, 10, 11
Affiliation
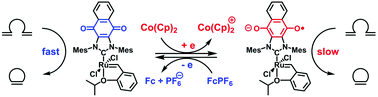
|
A Ru(II) complex ligated to a quinone-annulated N-heterocyclic carbene (NHC) was synthesized as a redox-active analogue of the Hoveyda–Grubbs II generation catalyst. The complex exhibited a single reversible reduction with a E1/2 of −0.63 V (vs. SCE), and was successfully reduced and then oxidized with high fidelity using chemical reagents. While the catalyst facilitated a range of ring-closing metathesis (RCM) reactions in its neutral state, its activity was inhibited upon the introduction of a suitable reducing reagent. A series of density functional theory calculations revealed that the differences in catalytic activity may be attributed to the stronger donating ability of the reduced NHC ligand which stabilized a ruthenacyclobutane intermediate and thus suppressed the rate-determining retro-[2 + 2] cycloaddition step of the underlying RCM mechanism.
中文翻译:

可氧化还原转换的闭环复分解催化剂
连接到醌型N-杂环卡宾(NHC)的Ru(II)络合物被合成为Hoveyda-Grubbs II生成催化剂的氧化还原活性类似物。该配合物表现出单一的可逆还原,E 1/2为-0.63 V(vs。SCE),并成功还原,然后使用化学试剂高保真度氧化。尽管催化剂在中性状态下促进了一系列的闭环复分解(RCM)反应,但在引入合适的还原剂后其活性受到了抑制。一系列密度泛函理论计算表明,催化活性的差异可能归因于还原的NHC配体的较强供体能力,该配体稳定了钌烷环丁烷中间体,从而抑制了速率确定型的逆[2 + 2]环加成步骤。潜在的RCM机制。
更新日期:2017-09-12
中文翻译:

可氧化还原转换的闭环复分解催化剂
连接到醌型N-杂环卡宾(NHC)的Ru(II)络合物被合成为Hoveyda-Grubbs II生成催化剂的氧化还原活性类似物。该配合物表现出单一的可逆还原,E 1/2为-0.63 V(vs。SCE),并成功还原,然后使用化学试剂高保真度氧化。尽管催化剂在中性状态下促进了一系列的闭环复分解(RCM)反应,但在引入合适的还原剂后其活性受到了抑制。一系列密度泛函理论计算表明,催化活性的差异可能归因于还原的NHC配体的较强供体能力,该配体稳定了钌烷环丁烷中间体,从而抑制了速率确定型的逆[2 + 2]环加成步骤。潜在的RCM机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号