当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and application of a bifunctional organocatalyst guided by electron density topological analyses
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-08-28 00:00:00 , DOI: 10.1039/c7cy00430c Eddy I. Jiménez 1, 2, 3, 4, 5 , Wilmer E. Vallejo Narváez 1, 2, 3, 4, 5 , Tomás Rocha-Rinza 1, 2, 3, 4, 5 , Marcos Hernández-Rodríguez 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-08-28 00:00:00 , DOI: 10.1039/c7cy00430c Eddy I. Jiménez 1, 2, 3, 4, 5 , Wilmer E. Vallejo Narváez 1, 2, 3, 4, 5 , Tomás Rocha-Rinza 1, 2, 3, 4, 5 , Marcos Hernández-Rodríguez 1, 2, 3, 4, 5
Affiliation
|
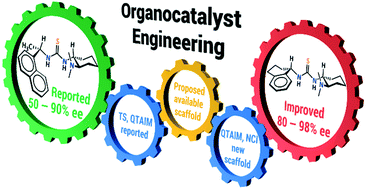
The design of new catalysts usually involves the synthesis and experimental evaluation of many compounds. We report herein an alternative methodology for designing bifunctional organocatalysts based on electron density topological analyses, i.e. the quantum theory of atoms in molecules and the non-covalent interaction index of the transition state (TS) of a Michael addition. These methods allowed us to identify key weak attractive interactions (CH⋯π) in the TS of a previously investigated catalyst. We sought a commercially available scaffold with the aromatic ring in the appropriate orientation to form such attractive interactions. We modelled the TS with this new scaffold and determined the occurrence of CH⋯π contacts by the aforementioned methodology. The new catalyst presented an excellent performance regardless of the substrate used in the reaction. Overall, this new approach shows how to exploit the quantum chemical topology as a tool to understand stereoselective reactions, successfully design bifunctional organocatalysts and avoid the synthesis of many catalysts.
中文翻译:

电子密度拓扑分析指导的双功能有机催化剂的设计与应用
新催化剂的设计通常涉及许多化合物的合成和实验评估。我们在此报告了一种基于电子密度拓扑分析设计双功能有机催化剂的替代方法,即分子中原子的量子理论和迈克尔加成跃迁状态(TS)的非共价相互作用指数。这些方法使我们能够确定先前研究过的催化剂的TS中关键的弱吸引相互作用(CH⋯π)。我们寻求一种在适当方向上具有芳香环的市售支架,以形成这种有吸引力的相互作用。我们用这种新型支架对TS进行建模,并通过上述方法确定了CH⋯π接触的发生。不论反应中使用何种底物,新催化剂均具有出色的性能。总的来说,这种新方法展示了如何利用量子化学拓扑学作为理解立体选择性反应的工具,成功设计双功能有机催化剂并避免合成许多催化剂的方法。
更新日期:2017-09-12
中文翻译:

电子密度拓扑分析指导的双功能有机催化剂的设计与应用
新催化剂的设计通常涉及许多化合物的合成和实验评估。我们在此报告了一种基于电子密度拓扑分析设计双功能有机催化剂的替代方法,即分子中原子的量子理论和迈克尔加成跃迁状态(TS)的非共价相互作用指数。这些方法使我们能够确定先前研究过的催化剂的TS中关键的弱吸引相互作用(CH⋯π)。我们寻求一种在适当方向上具有芳香环的市售支架,以形成这种有吸引力的相互作用。我们用这种新型支架对TS进行建模,并通过上述方法确定了CH⋯π接触的发生。不论反应中使用何种底物,新催化剂均具有出色的性能。总的来说,这种新方法展示了如何利用量子化学拓扑学作为理解立体选择性反应的工具,成功设计双功能有机催化剂并避免合成许多催化剂的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号