当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of electron transfer on the stability of hydrogen bonds
Chemical Science ( IF 7.6 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1039/c7sc03361c Tyler M. Porter 1, 2, 3, 4 , Gavin P. Heim 1, 2, 3, 4 , Clifford P. Kubiak 1, 2, 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1039/c7sc03361c Tyler M. Porter 1, 2, 3, 4 , Gavin P. Heim 1, 2, 3, 4 , Clifford P. Kubiak 1, 2, 3, 4
Affiliation
|
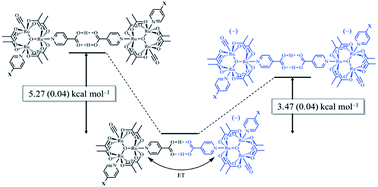
The measurement of the dimerization constants of hydrogen-bonded ruthenium complexes (12, 22, 32) linked by a self-complementary pair of 4-pyridylcarboxylic acid ligands in different redox states is reported. Using a combination of FTIR and UV/vis/NIR spectroscopies, the dimerization constants (KD) of the isovalent, neutral states, 12, 22, 32, were found to range from 75 to 130 M−1 (ΔG0 = −2.56 to −2.88 kcal mol−1), while the dimerization constants (K2−) of the isovalent, doubly-reduced states, (12)2−, (22)2−, (32)2−, were found to range from 2000 to 2500 M−1 (ΔG0 = −4.5 to −4.63 kcal mol−1). From the aforementioned values and the comproportionation constant for the mixed-valent dimers, the dimerization constants (KMV) of the mixed-valent, hydrogen-bonded dimers, (12)−, (22)−, (32)−, were found to range from 0.5 × 106 to 1.2 × 106 M−1 (ΔG0 = −7.78 to −8.31 kcal mol−1). On average, the hydrogen-bonded, mixed-valent states are stabilized by −5.27 (0.04) kcal mol−1 relative to the isovalent, neutral, hydrogen-bonded dimers and −3.47 (0.06) kcal mol−1 relative to the isovalent, dianionic hydrogen bonded dimers. Electron exchange in the mixed valence states imparts significant stability to hydrogen bonding. This is the first quantitative measurement of the strength of hydrogen bonds in the presence and absence of electronic exchange.
中文翻译:

电子转移对氢键稳定性的影响
(氢键钌络合物的二聚化常数的测量1 2,2 2,3 2)通过在不同的氧化还原状态的自互补对4- pyridylcarboxylic酸配位体连接的报道。使用FTIR和UV / VIS / NIR光谱的组合,二聚化常数(ķ d的等价的),中性状态,1 2,2 2,3 2,被发现范围从75到130米-1(Δ ģ 0 = -2.56至-2.88 kcal mol -1),而二聚化常数(K 2-)等价的双还原态(1 2)2-,(2 2)2-,(3 2)2-的)范围为2000到2500 M -1(ΔG 0 = -4.5到-4.63 kcal mol -1)。根据上述值和混合价二聚体的比例均衡常数,可以算出(1 2)-,(2 2)-,(3 2)-氢键合混合二聚体的二聚常数(K MV)。被发现的范围为0.5×10 6至1.2×10 6 M -1(ΔG 0= -7.78至-8.31kcal mol -1)。平均而言,相对于等价的中性氢键合二聚体,氢键合的混合价态稳定在−5.27(0.04)kcal mol -1和相对于等价的−3.47(0.06)kcal mol -1。双阴离子氢键二聚体。混合价态的电子交换赋予氢键显着的稳定性。这是在有电子交换和无电子交换的情况下,氢键强度的首次定量测量。
更新日期:2017-09-12
中文翻译:

电子转移对氢键稳定性的影响
(氢键钌络合物的二聚化常数的测量1 2,2 2,3 2)通过在不同的氧化还原状态的自互补对4- pyridylcarboxylic酸配位体连接的报道。使用FTIR和UV / VIS / NIR光谱的组合,二聚化常数(ķ d的等价的),中性状态,1 2,2 2,3 2,被发现范围从75到130米-1(Δ ģ 0 = -2.56至-2.88 kcal mol -1),而二聚化常数(K 2-)等价的双还原态(1 2)2-,(2 2)2-,(3 2)2-的)范围为2000到2500 M -1(ΔG 0 = -4.5到-4.63 kcal mol -1)。根据上述值和混合价二聚体的比例均衡常数,可以算出(1 2)-,(2 2)-,(3 2)-氢键合混合二聚体的二聚常数(K MV)。被发现的范围为0.5×10 6至1.2×10 6 M -1(ΔG 0= -7.78至-8.31kcal mol -1)。平均而言,相对于等价的中性氢键合二聚体,氢键合的混合价态稳定在−5.27(0.04)kcal mol -1和相对于等价的−3.47(0.06)kcal mol -1。双阴离子氢键二聚体。混合价态的电子交换赋予氢键显着的稳定性。这是在有电子交换和无电子交换的情况下,氢键强度的首次定量测量。







































 京公网安备 11010802027423号
京公网安备 11010802027423号