当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thioamide, a Hydrogen Bond Acceptor in Proteins and Nucleic Acids
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1021/acs.jpclett.7b01810 V. Rao Mundlapati 1, 2 , Sanjeev Gautam 1, 2 , Dipak Kumar Sahoo 1, 2 , Arindam Ghosh 1, 2 , Himansu S. Biswal 1, 2
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1021/acs.jpclett.7b01810 V. Rao Mundlapati 1, 2 , Sanjeev Gautam 1, 2 , Dipak Kumar Sahoo 1, 2 , Arindam Ghosh 1, 2 , Himansu S. Biswal 1, 2
Affiliation
|
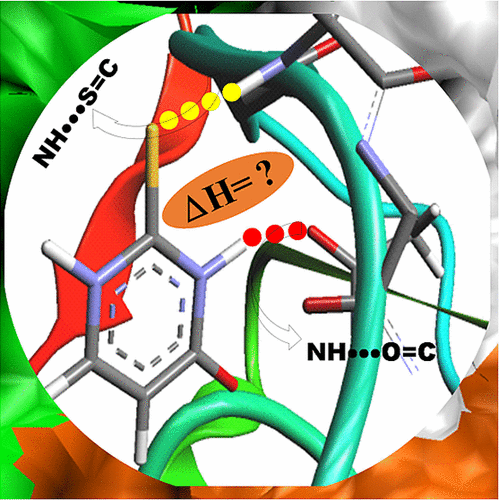
Thioamides are used as potential surrogates of amides to study the structure and dynamics of proteins and nucleic acids. However, incorporation of thioamides in biomolecules leads to changes in their structures and conformations mostly attributed to the strength of the amide–N–H···S═C hydrogen bond. In most cases, it is considered weak owing to the small electronegativity of sulfur, and in some cases, it is as strong as conventional H-bonds. Herein, adopting PDB structure analysis, NMR spectroscopy, and quantum chemistry calculations, we have shown that thioamides in a geometrical and structural constraint-free environment are capable of forming strong H-bonds like their amide counterparts. These studies also enabled us to determine the amide–N–H···S═C H-bond enthalpy (ΔH) very precisely. The estimated ΔH for the amide–N–H···S═C H-bond is ∼−30 kJ/mol, which suggests that the amide–N–H···S═C H-bond is a strong H-bond and merits its inclusion in computational force fields for biomolecular structure simulations to explore the role of amide–N–H···S═C H-bonds in nucleobase pairing and protein folding.
中文翻译:

硫酰胺,蛋白质和核酸中的氢键受体
硫酰胺被用作酰胺的潜在替代物,以研究蛋白质和核酸的结构和动力学。但是,在生物分子中掺入硫酰胺会导致其结构和构型发生变化,这主要归因于酰胺–NH–··S═C氢键的强度。在大多数情况下,由于硫的电负性小,它被认为是弱的,在某些情况下,它与常规的H键一样强。在本文中,采用PDB结构分析,NMR光谱和量子化学计算,我们已经证明,在几何和结构无约束的环境中,硫代酰胺能够像它们的酰胺对应物一样形成牢固的H键。这些研究还使我们能够非常精确地确定酰胺-N–H··S HC H键焓(ΔH)。估计的Δħ的酰胺N-H···S═CH-键是〜-30千焦/摩尔,这表明酰胺-N-H···S═CH-键是强氢键和值得将其包含在用于生物分子结构模拟的计算力场中,以探讨酰胺–NH–··S═CH键在核碱基配对和蛋白质折叠中的作用。
更新日期:2017-09-12
中文翻译:

硫酰胺,蛋白质和核酸中的氢键受体
硫酰胺被用作酰胺的潜在替代物,以研究蛋白质和核酸的结构和动力学。但是,在生物分子中掺入硫酰胺会导致其结构和构型发生变化,这主要归因于酰胺–NH–··S═C氢键的强度。在大多数情况下,由于硫的电负性小,它被认为是弱的,在某些情况下,它与常规的H键一样强。在本文中,采用PDB结构分析,NMR光谱和量子化学计算,我们已经证明,在几何和结构无约束的环境中,硫代酰胺能够像它们的酰胺对应物一样形成牢固的H键。这些研究还使我们能够非常精确地确定酰胺-N–H··S HC H键焓(ΔH)。估计的Δħ的酰胺N-H···S═CH-键是〜-30千焦/摩尔,这表明酰胺-N-H···S═CH-键是强氢键和值得将其包含在用于生物分子结构模拟的计算力场中,以探讨酰胺–NH–··S═CH键在核碱基配对和蛋白质折叠中的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号