当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ni2P(O)/Fe2P(O) Interface Can Boost Oxygen Evolution Electrocatalysis
ACS Energy Letters ( IF 19.3 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acsenergylett.7b00638 Peng Fei Liu 1 , Xu Li 1 , Shuang Yang 1 , Meng Yang Zu 1 , Porun Liu 2 , Bo Zhang 3 , Li Rong Zheng 4 , Huijun Zhao 2 , Hua Gui Yang 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acsenergylett.7b00638 Peng Fei Liu 1 , Xu Li 1 , Shuang Yang 1 , Meng Yang Zu 1 , Porun Liu 2 , Bo Zhang 3 , Li Rong Zheng 4 , Huijun Zhao 2 , Hua Gui Yang 1
Affiliation
|
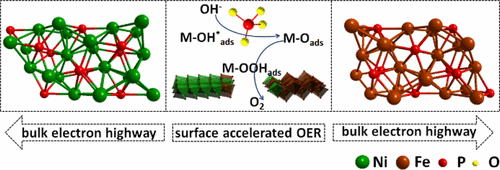
Oxygen evolution reaction (OER) plays a paramount role in renewable energy technologies. However, the slow kinetics of OER seriously limits the overall performance and commercialization. Here, we rationally design a metallic Ni2P/Fe2P interface, which can be in situ oxidized to a Ni2P(O)/Fe2P(O) interface to enhance OER efficiency, with active doped oxyhydroxides and phosphates on the surface and conductive phosphide in the bulk. The resulting catalysts require a low overpotential of 179 mV to achieve a current density of 10 mA/cm2 (without iR compensation) and can continuously drive OER for 120 h without any obvious degradation, which rivals most reported OER catalysts. These results suggest that we are able to design multicomponent metallic precatalysts to construct most active surface layers and conductive bulks, further boosting OER performance for real-world electrolysis utilization.
中文翻译:

Ni 2 P(O)/ Fe 2 P(O)界面可促进析氧电催化
氧气析出反应(OER)在可再生能源技术中起着至关重要的作用。但是,OER的动力学缓慢严重限制了整体性能和商业化。在这里,我们合理地设计了一个金属Ni 2 P / Fe 2 P界面,该界面可以被原位氧化为Ni 2 P(O)/ Fe 2 P(O)界面,以提高OER效率,并在表面活性掺杂羟基氧化物和磷酸盐主体中的表面和导电磷化物。所得催化剂需要179 mV的低超电势才能实现10 mA / cm 2的电流密度(无iR补偿),并且可以连续驱动OER 120小时而没有任何明显的降解,这可以与大多数报道的OER催化剂相媲美。这些结果表明,我们能够设计出多组分金属预催化剂来构造最活跃的表面层和导电块,从而进一步提高了OER在实际电解中的性能。
更新日期:2017-09-11
中文翻译:

Ni 2 P(O)/ Fe 2 P(O)界面可促进析氧电催化
氧气析出反应(OER)在可再生能源技术中起着至关重要的作用。但是,OER的动力学缓慢严重限制了整体性能和商业化。在这里,我们合理地设计了一个金属Ni 2 P / Fe 2 P界面,该界面可以被原位氧化为Ni 2 P(O)/ Fe 2 P(O)界面,以提高OER效率,并在表面活性掺杂羟基氧化物和磷酸盐主体中的表面和导电磷化物。所得催化剂需要179 mV的低超电势才能实现10 mA / cm 2的电流密度(无iR补偿),并且可以连续驱动OER 120小时而没有任何明显的降解,这可以与大多数报道的OER催化剂相媲美。这些结果表明,我们能够设计出多组分金属预催化剂来构造最活跃的表面层和导电块,从而进一步提高了OER在实际电解中的性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号