当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantum Chemical Prediction of Equilibrium Acidities of Ureas, Deltamides, Squaramides, and Croconamides
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acs.joc.7b02083 Junming Ho 1, 2 , Vincent E. Zwicker 2 , Karen K. Y. Yuen 2 , Katrina A. Jolliffe 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acs.joc.7b02083 Junming Ho 1, 2 , Vincent E. Zwicker 2 , Karen K. Y. Yuen 2 , Katrina A. Jolliffe 2
Affiliation
|
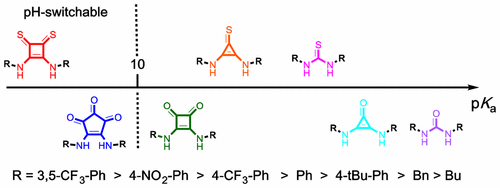
Robust quantum chemical methods are employed to predict the pKa’s of several families of dual hydrogen-bonding organocatalysts/anion receptors, including deltamides and croconamides as well as their thio derivatives. The average accuracy of these predictions is ∼1 pKa unit and allows for a comparison of the acidity between classes of receptors and for quantitative studies of substituent effects. These computational insights further explain the relationship between pKa and chloride anion affinity of these receptors that will be important for designing future anion receptors and organocatalysts.
中文翻译:

尿素,Deltamides,Squaramides和Croconamides的平衡酸度的量子化学预测
鲁棒的量子化学方法被用于预测几个氢键键合的有机催化剂/阴离子受体的几个家族的p K a,包括deltamides和croconamides及其硫代衍生物。这些预测的平均准确度约为1 p K a单位,可以比较各类受体之间的酸度,并可以定量研究取代基的作用。这些计算见解进一步解释了p K a和这些受体的氯阴离子亲和力之间的关系,这对于设计未来的阴离子受体和有机催化剂将是重要的。
更新日期:2017-09-11
中文翻译:

尿素,Deltamides,Squaramides和Croconamides的平衡酸度的量子化学预测
鲁棒的量子化学方法被用于预测几个氢键键合的有机催化剂/阴离子受体的几个家族的p K a,包括deltamides和croconamides及其硫代衍生物。这些预测的平均准确度约为1 p K a单位,可以比较各类受体之间的酸度,并可以定量研究取代基的作用。这些计算见解进一步解释了p K a和这些受体的氯阴离子亲和力之间的关系,这对于设计未来的阴离子受体和有机催化剂将是重要的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号