Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Adsorbed Alcohol Layers on the Behavior of Water Molecules Confined in a Graphene Nanoslit: A Molecular Dynamics Study
Langmuir ( IF 3.7 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acs.langmuir.7b02038 Qingwei Gao 1 , Yudan Zhu 1 , Yang Ruan 1 , Yumeng Zhang 1 , Wei Zhu 1 , Xiaohua Lu 1 , Linghong Lu 1
Langmuir ( IF 3.7 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acs.langmuir.7b02038 Qingwei Gao 1 , Yudan Zhu 1 , Yang Ruan 1 , Yumeng Zhang 1 , Wei Zhu 1 , Xiaohua Lu 1 , Linghong Lu 1
Affiliation
|
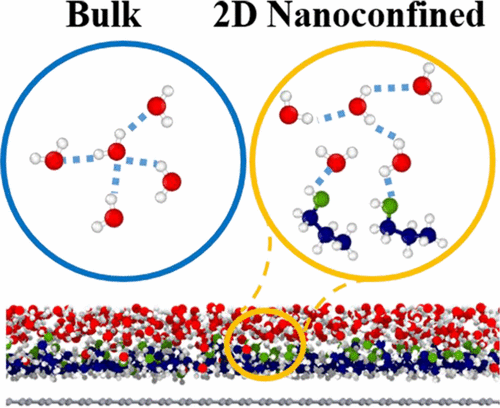
With the rapid development of a two-dimensional (2D) nanomaterial, the confined liquid binary mixture has attracted increasing attention, which has significant potential in membrane separation. Alcohol/water is one of the most common systems in liquid–liquid separation. As one of the most focused systems, recent studies have found that ethanol molecules were preferentially adsorbed on the inner surface of the pore wall and formed an adsorbed ethanol layer under 2D nanoconfinement. To evaluate the effect of the alcohol adsorption layer on the mobility of water molecules, molecular simulations were performed to investigate four types of alcohol/water binary mixtures confined under a 20 Å graphene slit. Residence times of the water molecules covering the alcohol layer were in the order of methanol/water < ethanol/water < 1-propanol/water < 1-butanol/water. Detailed microstructural analysis of the hydrogen bonding (H-bond) network elucidated the underlying mechanism on the molecular scale in which a small average number of H-bonds between the preferentially adsorbed alcohol molecules and the surrounding water molecules could induce a small degree of damage to the H-bond network of the water molecules covering the alcohol layer, resulting in the long residence time of the water molecules.
中文翻译:

醇层吸附对石墨烯纳米缝中水分子行为的影响:分子动力学研究
随着二维(2D)纳米材料的迅速发展,封闭的液态二元混合物引起了越来越多的关注,这在膜分离中具有巨大的潜力。酒精/水是液-液分离中最常见的系统之一。作为最受关注的系统之一,最近的研究发现,乙醇分子优先吸附在孔壁的内表面上,并在二维纳米约束下形成了吸附的乙醇层。为了评估醇吸附层对水分子迁移率的影响,进行了分子模拟以研究限制在20Å石墨烯缝隙下的四种类型的醇/水二元混合物。覆盖醇层的水分子的停留时间为甲醇/水<乙醇/水<1-丙醇/水< 1-丁醇/水。氢键(H-bond)网络的详细微观结构分析阐明了分子尺度上的潜在机理,其中优先吸附的醇分子与周围水分子之间的平均数量很少的H-键可能会导致对水分子的H键网络覆盖醇层,导致水分子的停留时间长。
更新日期:2017-09-11
中文翻译:

醇层吸附对石墨烯纳米缝中水分子行为的影响:分子动力学研究
随着二维(2D)纳米材料的迅速发展,封闭的液态二元混合物引起了越来越多的关注,这在膜分离中具有巨大的潜力。酒精/水是液-液分离中最常见的系统之一。作为最受关注的系统之一,最近的研究发现,乙醇分子优先吸附在孔壁的内表面上,并在二维纳米约束下形成了吸附的乙醇层。为了评估醇吸附层对水分子迁移率的影响,进行了分子模拟以研究限制在20Å石墨烯缝隙下的四种类型的醇/水二元混合物。覆盖醇层的水分子的停留时间为甲醇/水<乙醇/水<1-丙醇/水< 1-丁醇/水。氢键(H-bond)网络的详细微观结构分析阐明了分子尺度上的潜在机理,其中优先吸附的醇分子与周围水分子之间的平均数量很少的H-键可能会导致对水分子的H键网络覆盖醇层,导致水分子的停留时间长。











































 京公网安备 11010802027423号
京公网安备 11010802027423号