当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Endocannabinoid Interaction with Human FABP1: Impact of the T94A Variant
Biochemistry ( IF 2.9 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acs.biochem.7b00647 Gregory G. Martin 1 , Huan Huang 1 , Avery L. McIntosh 1 , Ann B. Kier 2 , Friedhelm Schroeder 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acs.biochem.7b00647 Gregory G. Martin 1 , Huan Huang 1 , Avery L. McIntosh 1 , Ann B. Kier 2 , Friedhelm Schroeder 1
Affiliation
|
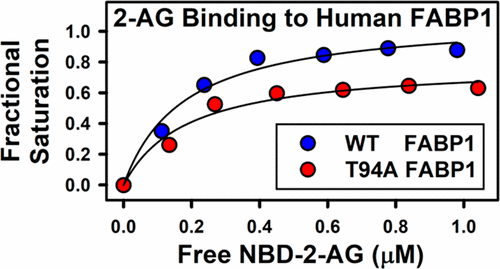
Using recombinant human wild-type fatty acid binding protein 1 (WT FABP1 T94T) and a variant (FABP1 T94A) protein, fluorescence binding assays, and circular dichroism, it was shown for the first time that WT FABP1 and the T94A variant each have a single, relatively hydrophobic site for binding fluorescent NBD-labeled analogues of N-arachidonoylethanolamide and 2-arachidonoylglycerol with high affinity. Most native N-acylethanolamides (NAEs) but only one 2-monoacylglycerol [i.e., 2-arachidonoylglycerol (2-AG)] displaced WT FABP1-bound fluorescently labeled endocannabinoids (ECs). While the T94A variant did not differ in affinity for AEA and most other NAEs, it exhibited a modestly higher affinity for OEA, as well as a higher affinity for 2-AG. Binding of AEA and 2-AG altered WT FABP1’s secondary structure more extensively than any other previously examined ligand did. The T94A variant without a ligand was more susceptible to temperature-induced unfolding. While the T94A variant was much less sensitive to ligand (i.e., AEA or 2-AG)-induced conformational change, nevertheless binding of AEA and 2-AG significantly stabilized the T94A structure to thermal unfolding. These data provide the first evidence that ECs not only bind to but also alter the secondary structure of the human FABP1, with the latter markedly impacted by the T94A substitution, a variant strongly associated with hepatic accumulation of lipids and non-alcoholic fatty liver disease (NAFLD). Importantly, NAFLD has been associated with elevated hepatic levels of ECs and FABP1.
中文翻译:

内源性大麻素与人FABP1的相互作用:T94A变异体的影响
使用重组人野生型脂肪酸结合蛋白1(WT FABP1 T94T)和变体(FABP1 T94A)蛋白,荧光结合测定法和圆二色性,首次证明WT FABP1和T94A变体各自具有一个相对疏水的位点,用于以高亲和力结合荧光NBD标记的N-花生四烯酰基乙醇酰胺和2-花生四烯酰基甘油的类似物。最原生的N-酰基乙醇酰胺(NAE),但只有一种2-单酰基甘油[即2-花生四烯酰基甘油(2-AG)]取代了WT FABP1结合的荧光标记的内源性大麻素(EC)。尽管T94A变体对AEA和大多数其他NAE的亲和力没有差异,但对OEA的适度更高,对2-AG的亲和力更高。AEA和2-AG的结合比以前检查过的任何其他配体更广泛地改变了WT FABP1的二级结构。没有配体的T94A变体更容易受到温度诱导的解折叠。尽管T94A变体对配体(即AEA或2-AG)诱导的构象变化不那么敏感,但是AEA和2-AG的结合显着稳定了T94A结构对热展开的稳定性。这些数据提供了第一个证据,表明EC不仅与人FABP1结合,而且还改变了人FABP1的二级结构,后者显着受到T94A替代的影响,T94A替代是与肝脂质蓄积和非酒精性脂肪肝疾病密切相关的变体( NAFLD)。重要的是,NAFLD与肝细胞ECs和FABP1的升高有关。
更新日期:2017-09-11
中文翻译:

内源性大麻素与人FABP1的相互作用:T94A变异体的影响
使用重组人野生型脂肪酸结合蛋白1(WT FABP1 T94T)和变体(FABP1 T94A)蛋白,荧光结合测定法和圆二色性,首次证明WT FABP1和T94A变体各自具有一个相对疏水的位点,用于以高亲和力结合荧光NBD标记的N-花生四烯酰基乙醇酰胺和2-花生四烯酰基甘油的类似物。最原生的N-酰基乙醇酰胺(NAE),但只有一种2-单酰基甘油[即2-花生四烯酰基甘油(2-AG)]取代了WT FABP1结合的荧光标记的内源性大麻素(EC)。尽管T94A变体对AEA和大多数其他NAE的亲和力没有差异,但对OEA的适度更高,对2-AG的亲和力更高。AEA和2-AG的结合比以前检查过的任何其他配体更广泛地改变了WT FABP1的二级结构。没有配体的T94A变体更容易受到温度诱导的解折叠。尽管T94A变体对配体(即AEA或2-AG)诱导的构象变化不那么敏感,但是AEA和2-AG的结合显着稳定了T94A结构对热展开的稳定性。这些数据提供了第一个证据,表明EC不仅与人FABP1结合,而且还改变了人FABP1的二级结构,后者显着受到T94A替代的影响,T94A替代是与肝脂质蓄积和非酒精性脂肪肝疾病密切相关的变体( NAFLD)。重要的是,NAFLD与肝细胞ECs和FABP1的升高有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号