当前位置:
X-MOL 学术
›
JAMA Intern. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Research and Development Spending to Bring a Single Cancer Drug to Market and Revenues After Approval
JAMA Internal Medicine ( IF 39.0 ) Pub Date : 2017-11-01 , DOI: 10.1001/jamainternmed.2017.3601 Vinay Prasad 1, 2, 3 , Sham Mailankody 4, 5
JAMA Internal Medicine ( IF 39.0 ) Pub Date : 2017-11-01 , DOI: 10.1001/jamainternmed.2017.3601 Vinay Prasad 1, 2, 3 , Sham Mailankody 4, 5
Affiliation
|
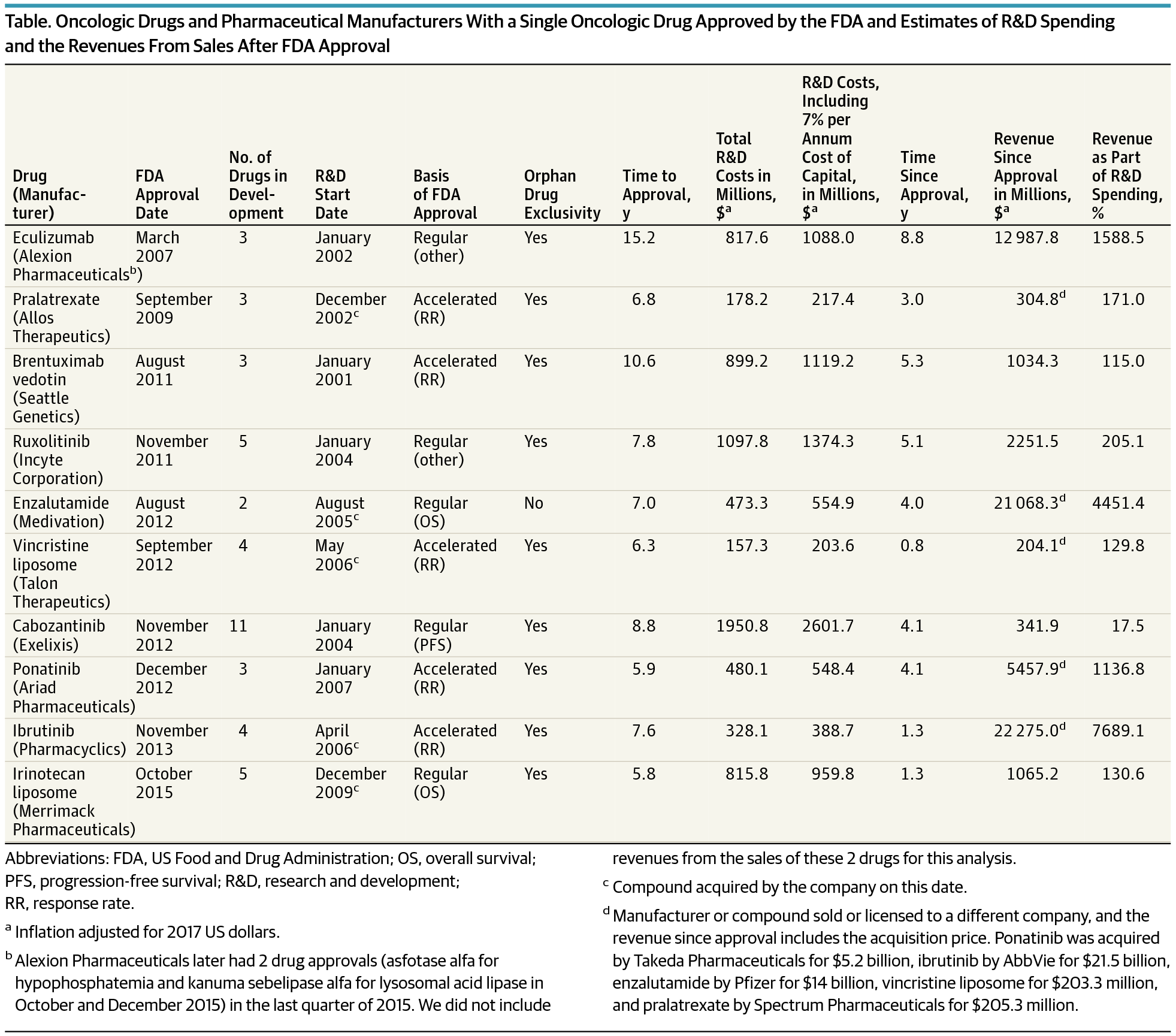
Importance A common justification for high cancer drug prices is the sizable research and development (R&D) outlay necessary to bring a drug to the US market. A recent estimate of R&D spending is $2.7 billion (2017 US dollars). However, this analysis lacks transparency and independent replication. Objective To provide a contemporary estimate of R&D spending to develop cancer drugs. Design, Setting, and Participants Analysis of US Securities and Exchange Commission filings for drug companies with no drugs on the US market that received approval by the US Food and Drug Administration for a cancer drug from January 1, 2006, through December 31, 2015. Cumulative R&D spending was estimated from initiation of drug development activity to date of approval. Earnings were also identified from the time of approval to the present. The study was conducted from December 10, 2016, to March 2, 2017. Main Outcomes and Measures Median R&D spending on cancer drug development. Results Ten companies and drugs were included in this analysis. The 10 companies had a median time to develop a drug of 7.3 years (range, 5.8-15.2 years). Five drugs (50%) received accelerated approval from the US Food and Drug Administration, and 5 (50%) received regular approval. The median cost of drug development was $648.0 million (range, $157.3 million to $1950.8 million). The median cost was $757.4 million (range, $203.6 million to $2601.7 million) for a 7% per annum cost of capital (or opportunity costs) and $793.6 million (range, $219.1 million to $2827.1 million) for a 9% opportunity costs. With a median of 4.0 years (range, 0.8-8.8 years) since approval, the total revenue from sales of these 10 drugs since approval was $67.0 billion compared with total R&D spending of $7.2 billion ($9.1 billion, including 7% opportunity costs). Conclusions and Relevance The cost to develop a cancer drug is $648.0 million, a figure significantly lower than prior estimates. The revenue since approval is substantial (median, $1658.4 million; range, $204.1 million to $22 275.0 million). This analysis provides a transparent estimate of R&D spending on cancer drugs and has implications for the current debate on drug pricing.
中文翻译:

将单一抗癌药物推向市场的研发支出和批准后的收入
重要性 抗癌药物价格居高不下的一个常见理由是将药物推向美国市场所需的大量研发 (R&D) 支出。最近对研发支出的估计为 27 亿美元(2017 年美元)。然而,这种分析缺乏透明度和独立复制。目的提供对开发抗癌药物的研发支出的当代估计。2006 年 1 月 1 日至 2015 年 12 月 31 日期间美国食品和药物管理局批准在美国市场上没有药物的制药公司的美国证券交易委员会文件的设计、设置和参与者分析。累计研发支出是从药物开发活动开始到批准之日估算的。还确定了从批准时到现在的收益。该研究于2016年12月10日至2017年3月2日进行。 主要结果和措施 用于癌症药物开发的研发支出中位数。结果 本次分析包括 10 家公司和药品。这 10 家公司开发药物的中位时间为 7.3 年(范围为 5.8-15.2 年)。5 种药物(50%)获得了美国食品药品监督管理局的加速批准,5 种(50%)获得了常规批准。药物开发的中位成本为 6.48 亿美元(范围为 1.573 亿美元至 19.508 亿美元)。对于每年 7% 的资本成本(或机会成本)和 7.936 亿美元(范围,2.191 亿美元到 28.271 亿美元)的机会成本,平均成本为 7.574 亿美元(范围为 2.036 亿美元至 26.017 亿美元)。自批准以来的中位数为 4.0 年(范围,0.8-8.8 年),自批准以来,这 10 种药物的销售总收入为 670 亿美元,而研发总支出为 72 亿美元(91 亿美元,包括 7% 的机会成本)。结论和相关性 开发抗癌药物的成本为 6.48 亿美元,这一数字大大低于之前的估计。自批准以来的收入相当可观(中位数为 16.584 亿美元;范围为 2.041 亿美元至 222.75 亿美元)。该分析提供了对抗癌药物研发支出的透明估计,并对当前关于药物定价的辩论产生了影响。自批准以来的收入相当可观(中位数为 16.584 亿美元;范围为 2.041 亿美元至 222.75 亿美元)。该分析提供了对抗癌药物研发支出的透明估计,并对当前关于药物定价的辩论产生了影响。自批准以来的收入相当可观(中位数为 16.584 亿美元;范围为 2.041 亿美元至 222.75 亿美元)。该分析提供了对抗癌药物研发支出的透明估计,并对当前关于药物定价的辩论产生了影响。
更新日期:2017-11-01
中文翻译:

将单一抗癌药物推向市场的研发支出和批准后的收入
重要性 抗癌药物价格居高不下的一个常见理由是将药物推向美国市场所需的大量研发 (R&D) 支出。最近对研发支出的估计为 27 亿美元(2017 年美元)。然而,这种分析缺乏透明度和独立复制。目的提供对开发抗癌药物的研发支出的当代估计。2006 年 1 月 1 日至 2015 年 12 月 31 日期间美国食品和药物管理局批准在美国市场上没有药物的制药公司的美国证券交易委员会文件的设计、设置和参与者分析。累计研发支出是从药物开发活动开始到批准之日估算的。还确定了从批准时到现在的收益。该研究于2016年12月10日至2017年3月2日进行。 主要结果和措施 用于癌症药物开发的研发支出中位数。结果 本次分析包括 10 家公司和药品。这 10 家公司开发药物的中位时间为 7.3 年(范围为 5.8-15.2 年)。5 种药物(50%)获得了美国食品药品监督管理局的加速批准,5 种(50%)获得了常规批准。药物开发的中位成本为 6.48 亿美元(范围为 1.573 亿美元至 19.508 亿美元)。对于每年 7% 的资本成本(或机会成本)和 7.936 亿美元(范围,2.191 亿美元到 28.271 亿美元)的机会成本,平均成本为 7.574 亿美元(范围为 2.036 亿美元至 26.017 亿美元)。自批准以来的中位数为 4.0 年(范围,0.8-8.8 年),自批准以来,这 10 种药物的销售总收入为 670 亿美元,而研发总支出为 72 亿美元(91 亿美元,包括 7% 的机会成本)。结论和相关性 开发抗癌药物的成本为 6.48 亿美元,这一数字大大低于之前的估计。自批准以来的收入相当可观(中位数为 16.584 亿美元;范围为 2.041 亿美元至 222.75 亿美元)。该分析提供了对抗癌药物研发支出的透明估计,并对当前关于药物定价的辩论产生了影响。自批准以来的收入相当可观(中位数为 16.584 亿美元;范围为 2.041 亿美元至 222.75 亿美元)。该分析提供了对抗癌药物研发支出的透明估计,并对当前关于药物定价的辩论产生了影响。自批准以来的收入相当可观(中位数为 16.584 亿美元;范围为 2.041 亿美元至 222.75 亿美元)。该分析提供了对抗癌药物研发支出的透明估计,并对当前关于药物定价的辩论产生了影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号