当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalysis of Cross-Coupling and Homocoupling Reactions of Aryl Halides Utilizing Ni(0), Ni(I), and Ni(II) Precursors; Ni(0) Compounds as the Probable Catalytic Species but Ni(I) Compounds as Intermediates and Products
Organometallics ( IF 2.5 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acs.organomet.7b00446 Adeela Manzoor 1 , Patrick Wienefeld 1 , Michael C. Baird 1 , Peter H. M. Budzelaar 2
Organometallics ( IF 2.5 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acs.organomet.7b00446 Adeela Manzoor 1 , Patrick Wienefeld 1 , Michael C. Baird 1 , Peter H. M. Budzelaar 2
Affiliation
|
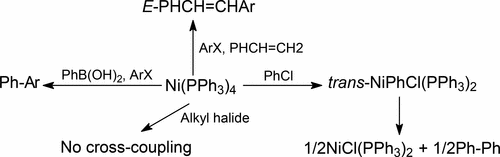
Both Ni(0) and Ni(I) compounds are believed to exhibit cross-coupling catalytic properties under various conditions, and the compounds Ni(PPh3)4 and NiCl(PPh3)3 are compared as catalysts for representative Suzuki–Miyaura and Heck–Mizoroki cross-coupling reactions. The Ni(0) compound exhibits catalytic activities, for cross-coupling of chloro and bromoanisole with phenylboronic acid and of bromobenzene with styrene, yielding results which are comparable with those of many palladium-based catalysts, but our findings with NiCl(PPh3)3 are at this point unclear. It seems to convert to catalytically active Ni(0) species under Suzuki–Miyaura reaction conditions and is ineffective for Heck–Mizoroki cross-coupling. The paramagnetic Ni(I) compounds NiX(PPh3)3 (X = Cl, Br, I) are characterized for the first time by 1H NMR spectroscopy and are found to exhibit broad meta and para resonances at δ 9–11 and 3–4, respectively, and very broad ortho resonances at δ 4−6; these resonances are very useful for detecting Ni(I) species in solution. The chemical shifts of NiCl(PPh3)3 vary with the concentration of free PPh3, with which it exchanges, and are also temperature-dependent, consistent with Curie law behavior. The compound trans-NiPhCl(PPh3)2, the product of oxidative addition of chlorobenzene to Ni(PPh3)4 and a putative intermediate in cross-coupling reactions of chlorobenzene, is found during the course of this investigation to exhibit entirely unanticipated thermal lability in solution in the absence of free PPh3. It readily decomposes to biphenyl and NiCl(PPh3)2 in a reaction relevant to the long-known but little-understood nickel-catalyzed conversion of aryl halides to biaryls. Ni(I) and biphenyl formation is initiated by PPh3 dissociation from trans-NiPhCl(PPh3)2 and formation of a dinuclear intermediate, a process which is now better defined using DFT methodologies.
中文翻译:

利用Ni(0),Ni(I)和Ni(II)前体催化芳基卤化物的交叉偶联和均偶联反应;Ni(0)化合物可能作为催化物质,但Ni(I)化合物作为中间体和产物
据信,Ni(0)和Ni(I)化合物在各种条件下均表现出交叉偶联的催化性能,并且比较了化合物Ni(PPh 3)4和NiCl(PPh 3)3作为代表性铃木–宫浦和Heck-Mizoroki交叉偶联反应。Ni(0)化合物具有催化活性,可用于氯和溴代苯甲醚与苯基硼酸的交叉偶联以及溴代苯与苯乙烯的交叉偶联,其结果可与许多钯基催化剂相媲美,但我们对NiCl(PPh 3)的发现3在这一点上还不清楚。在Suzuki-Miyaura反应条件下,它似乎转化为具有催化活性的Ni(0)物种,并且对于Heck-Mizoroki交叉偶联无效。顺磁性Ni(I)化合物NiX(PPh 3)3(X = Cl,Br,I)首次通过1 H NMR光谱表征,发现在δ9-11和3处表现出宽的间位和对位共振分别为–4和δ4-6处的非常宽的正交共振;这些共振对于检测溶液中的Ni(I)种类非常有用。NiCl(PPh 3)3的化学位移随游离PPh 3的浓度而变化与居里定律的行为保持一致,并且与之进行交换,并且还与温度有关。在该研究过程中发现化合物反式-NiPhCl(PPh 3)2,是氯苯氧化成Ni(PPh 3)4的产物,是假定的中间体,它是氯苯交叉偶联反应的中间产物,表现出完全出乎意料的热在没有游离PPh 3的情况下溶液的不稳定性。在与已知但很少了解的镍催化的芳基卤化物向联芳基的转化有关的反应中,它很容易分解为联苯和NiCl(PPh 3)2。Ni(I)和联苯的形成是由PPh引发的3从反式-NiPhCl(PPh 3)2解离并形成双核中间体,现在可以使用DFT方法更好地定义该过程。
更新日期:2017-09-11
中文翻译:

利用Ni(0),Ni(I)和Ni(II)前体催化芳基卤化物的交叉偶联和均偶联反应;Ni(0)化合物可能作为催化物质,但Ni(I)化合物作为中间体和产物
据信,Ni(0)和Ni(I)化合物在各种条件下均表现出交叉偶联的催化性能,并且比较了化合物Ni(PPh 3)4和NiCl(PPh 3)3作为代表性铃木–宫浦和Heck-Mizoroki交叉偶联反应。Ni(0)化合物具有催化活性,可用于氯和溴代苯甲醚与苯基硼酸的交叉偶联以及溴代苯与苯乙烯的交叉偶联,其结果可与许多钯基催化剂相媲美,但我们对NiCl(PPh 3)的发现3在这一点上还不清楚。在Suzuki-Miyaura反应条件下,它似乎转化为具有催化活性的Ni(0)物种,并且对于Heck-Mizoroki交叉偶联无效。顺磁性Ni(I)化合物NiX(PPh 3)3(X = Cl,Br,I)首次通过1 H NMR光谱表征,发现在δ9-11和3处表现出宽的间位和对位共振分别为–4和δ4-6处的非常宽的正交共振;这些共振对于检测溶液中的Ni(I)种类非常有用。NiCl(PPh 3)3的化学位移随游离PPh 3的浓度而变化与居里定律的行为保持一致,并且与之进行交换,并且还与温度有关。在该研究过程中发现化合物反式-NiPhCl(PPh 3)2,是氯苯氧化成Ni(PPh 3)4的产物,是假定的中间体,它是氯苯交叉偶联反应的中间产物,表现出完全出乎意料的热在没有游离PPh 3的情况下溶液的不稳定性。在与已知但很少了解的镍催化的芳基卤化物向联芳基的转化有关的反应中,它很容易分解为联苯和NiCl(PPh 3)2。Ni(I)和联苯的形成是由PPh引发的3从反式-NiPhCl(PPh 3)2解离并形成双核中间体,现在可以使用DFT方法更好地定义该过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号