当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Naphtho-p-quinodimethane Exhibiting Baird’s (Anti)Aromaticity, Broken Symmetry, and Attractive Photoluminescence
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acs.joc.7b01647 Siamak Shokri 1 , Jingbai Li 1 , Manoj K. Manna 1 , Gary P. Wiederrecht 2 , David J. Gosztola 2 , Angel Ugrinov 3 , Steffen Jockusch 4 , Andrey Yu Rogachev 1 , A. Jean-Luc Ayitou 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acs.joc.7b01647 Siamak Shokri 1 , Jingbai Li 1 , Manoj K. Manna 1 , Gary P. Wiederrecht 2 , David J. Gosztola 2 , Angel Ugrinov 3 , Steffen Jockusch 4 , Andrey Yu Rogachev 1 , A. Jean-Luc Ayitou 1
Affiliation
|
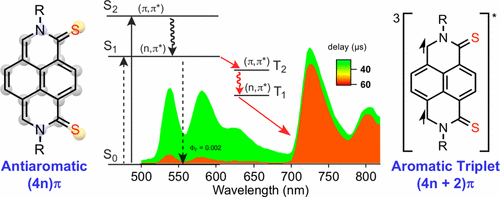
We report a novel reductive desulfurization reaction involving π-acidic naphthalene diimides (NDI) 1 using thionating agents such as Lawesson’s reagent. Along with the expected thionated NDI derivatives 2–6, new heterocyclic naphtho-p-quinodimethane compounds 7 depicting broken/reduced symmetry were successfully isolated and fully characterized. Empirical studies and theoretical modeling suggest that 7 was formed via a six-membered ring oxathiaphosphenine intermediate rather than the usual four-membered ring oxathiaphosphetane of 2–6. Aside from the reduced symmetry in 7 as confirmed by single-crystal XRD analysis, we established that the ground state UV–vis absorption of 7 is red-shifted in comparison to the parent NDI 1. This result was expected in the case of thionated polycyclic diimides. However, unusual low energy transitions originate from Baird 4nπ aromaticity of compounds 7 in lieu of the intrinsic Hückel (4n + 2)π aromaticity as encountered in NDI 1. Moreover, complementary theoretical modeling results also corroborate this change in aromaticity of 7. Consequently, photophysical investigations show that, compared to parent NDI 1, 7 can easily access and emit from its T1 state with a phosphorescence 3(7a)* lifetime of τP = 395 μs at 77 K indicative of the formation of the corresponding “aromatic triplet” species according to the Baird’s rule of aromaticity.
中文翻译:

Naphtho- p- quinodimethane甲烷,具有Baird的(反)芳香性,对称性破坏和有吸引力的光致发光
我们报告了一种新型的还原性脱硫反应,其中涉及使用硫代试剂(如Lawesson试剂)的π-酸性萘二酰亚胺(NDI)1。随着预期NDI thionated衍生物2 - 6,新的杂环萘p -quinodimethane化合物7描绘破碎/缩小对称性被成功分离并充分表征。经验研究和理论模型表明,7是通过一个六元环oxathiaphosphenine中间,而不是通常的四元环oxathiaphosphetane形成2 - 6。除了对称性降低7正如通过单晶XRD分析所证实的,我们确定与母体NDI 1相比,基态的UV-vis吸收7发生了红移。在亚硫酰化的多环二酰亚胺的情况下可以预期该结果。但是,异常的低能跃迁起源于化合物7的Baird4nπ芳香性,而不是NDI 1中遇到的固有Hückel(4n + 2)π芳香性。此外,互补的理论建模结果也证实了7的芳香性变化。因此,光物理研究表明,与母体NDI 1相比,7可以很容易地进入和发射其T 1用磷光状态3(图7A)*τ的寿命P在77K表示根据芳香性Baird的规则对应的“芳族三联体”物种形成的= 395微秒。
更新日期:2017-09-11
中文翻译:

Naphtho- p- quinodimethane甲烷,具有Baird的(反)芳香性,对称性破坏和有吸引力的光致发光
我们报告了一种新型的还原性脱硫反应,其中涉及使用硫代试剂(如Lawesson试剂)的π-酸性萘二酰亚胺(NDI)1。随着预期NDI thionated衍生物2 - 6,新的杂环萘p -quinodimethane化合物7描绘破碎/缩小对称性被成功分离并充分表征。经验研究和理论模型表明,7是通过一个六元环oxathiaphosphenine中间,而不是通常的四元环oxathiaphosphetane形成2 - 6。除了对称性降低7正如通过单晶XRD分析所证实的,我们确定与母体NDI 1相比,基态的UV-vis吸收7发生了红移。在亚硫酰化的多环二酰亚胺的情况下可以预期该结果。但是,异常的低能跃迁起源于化合物7的Baird4nπ芳香性,而不是NDI 1中遇到的固有Hückel(4n + 2)π芳香性。此外,互补的理论建模结果也证实了7的芳香性变化。因此,光物理研究表明,与母体NDI 1相比,7可以很容易地进入和发射其T 1用磷光状态3(图7A)*τ的寿命P在77K表示根据芳香性Baird的规则对应的“芳族三联体”物种形成的= 395微秒。










































 京公网安备 11010802027423号
京公网安备 11010802027423号