Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-09-09 , DOI: 10.1016/j.tetlet.2017.09.014 Alexander S. Kulikov , Margarita A. Epishina , Leonid L. Fershtat , Anna A. Romanova , Nina N. Makhova
|
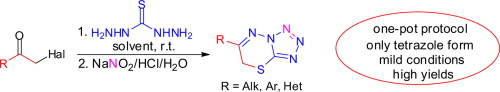
A new, simple, and general method for the synthesis of 6-R-7H-tetrazolo[5,1-b][1,3,4]thiadiazines (R = Ar, Het, Alk) has been developed. The described method is based on the one-pot condensation of α-haloketones with thiocarbohydrazide, nitrosation of the formed hydrazinylthiadiazine using NaNO2/HCl, and intramolecular cyclization of the nitrosation product via azide-tetrazole tautomerism. Spectroscopic and structural investigations revealed that the azide-tetrazole equilibrium is completely shifted to the tetrazole form both in solution and the solid state.
中文翻译:

通过一锅缩合/亚硝化/叠氮化物-四唑互变异构反应顺序有效合成6-取代的7 H-四唑[5,1- b ] [1,3,4]噻二嗪
已开发出一种新颖,简单且通用的合成6-R-7 H-四唑[5,1- b ] [1,3,4]噻二嗪(R = Ar,Het,Alk)的方法。所描述的方法基于α-卤代酮与硫代碳酰肼的一锅缩合,使用NaNO 2 / HCl对形成的肼基噻二嗪进行亚硝化,以及通过叠氮化物-四唑互变异构对亚硝化产物进行分子内环化。光谱和结构研究表明,叠氮化物-四唑平衡在溶液和固态下都完全转变为四唑形式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号