Journal of Catalysis ( IF 6.5 ) Pub Date : 2017-09-09 , DOI: 10.1016/j.jcat.2017.07.016 Rong-Zhen Liao , Per E.M. Siegbahn
|
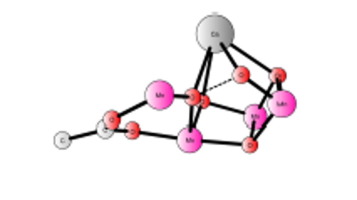
The laboratory synthesis of complexes to mimic the structure of the Mn4Ca cluster in the oxygen-evolving complex (OEC) of photosystem II is a very challenging task to accomplish. The most encouraging breakthrough in this field was recently achieved with the synthesis of a Mn4Ca complex (Zhang et al., 2015) that shows a very similar core structure to the OEC. On the basis of density functional calculations, the structure and the redox potentials of this Mn4Ca complex in acetonitrile are obtained with very good agreement to experiments. A possible mechanism for water oxidation is more problematic. If only the thermodynamics is considered and assuming a standard state of 1 mol/L, it turns out that up to five water molecules can be inserted into the complex with only a small cost. This leads to a barrier for OO bond formation which is 22.8 kcal/mol with an applied potential of 1.3 V. However, a study of the kinetics for the insertion of the critical water bridge between Mn3 and Mn4 indicates that the barrier for that process is quite high with 24.6 kcal/mol. A model where this water is not inserted also led to a rather high barrier for O
O bond formation with 31.7 kcal/mol with an applied potential of 1.3 V. However, the barrier decreases significantly to only 13.4 kcal/mol with an applied potential of 1.7 V. The different barrier originates from the different energetic penalty for the formation of the catalytic competent S4 state. A major experimental problem discussed below, is the instability of the complex, which does not allow a high water concentration. The best calculated overall mechanism obtained is essentially the same as the leading suggestion for the OEC, where the critical O
O bond formation takes place at the S4 state (formally Mn4IV,IV,IV,V) via direct coupling of a MnIV-oxyl radical and a di-Mn bridging oxo group.
中文翻译:

最近合成的Mn 4 Ca络合物的可能水缔合和氧化机理
模拟光系统II的析氧复合物(OEC)中的Mn 4 Ca团簇结构的复合物的实验室合成是一项非常艰巨的任务。最近,该领域最令人鼓舞的突破是合成了Mn 4 Ca络合物(Zhang等,2015),该络合物的核心结构与OEC非常相似。根据密度泛函计算,该Mn 4的结构和氧化还原电势获得的乙腈中的Ca络合物与实验非常吻合。水氧化的可能机理更加成问题。如果仅考虑热力学,并假设标准状态为1 mol / L,那么事实证明,只需花费很少的成本,即可将多达五个水分子插入到该配合物中。这导致形成O O键的势垒为22.8 kcal / mol,施加的电势为1.3V。但是,对在Mn3和Mn4之间插入临界水桥的动力学的研究表明,该过程的势垒24.6 kcal / mol是相当高的。没有插入这种水的模型也导致了对O的相当高的阻挡
在施加1.3 V的电势时形成31.7 kcal / mol的O键。但是,在施加1.7 V的势垒时,势垒显着降低至仅13.4 kcal / mol。催化能级为S 4状态。下文讨论的主要实验问题是配合物的不稳定性,不允许高水浓度。计算得出的最佳总体机理与针对OEC的主要建议基本相同,在OEC中,通过直接偶联Mn
在S 4状态(正式为Mn 4 IV,IV,IV,V)形成关键的O O键。IV-氧基和二-Mn桥接氧代基团。











































 京公网安备 11010802027423号
京公网安备 11010802027423号