当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of a Drug Delivery System Based on Chitosan Nanoparticles for Oral Administration of Interferon-α
Biomacromolecules ( IF 6.2 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.biomac.7b00959 Camila Cánepa , Julieta C. Imperiale , Carolina A. Berini , Marianela Lewicki , Alejandro Sosnik 1 , Mirna M. Biglione
Biomacromolecules ( IF 6.2 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.biomac.7b00959 Camila Cánepa , Julieta C. Imperiale , Carolina A. Berini , Marianela Lewicki , Alejandro Sosnik 1 , Mirna M. Biglione
Affiliation
|
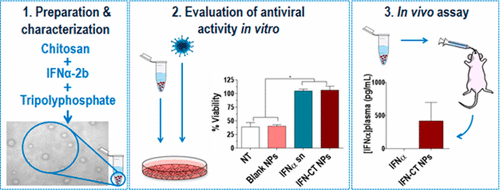
Despite the good clinical efficacy of interferon-alpha (IFNα) to treat some types of cancer and viral infections, this biological drug is underused given its severe adverse effects and high dosing parenteral regimens. Aiming to achieve a breakthrough in therapy with IFNα, this work reports for the first time on the design and full characterization of a novel nanomedicine of IFNα-2b-loaded chitosan nanoparticles (IFN-CT NPs) for oral delivery. IFN-CT NPs produced by ionotropic gelation, encapsulating approximately 100% of the drug, showed a size of 36 ± 8 nm, zeta potential of +30 mV (dynamic light scattering), and spherical morphology (transmission electron microscopy). The antiviral activity of IFN-CT NPs in vitro was comparable to that of commercial IFNα. Remarkably, both treatments stimulated the expression of IFN response genes to a similar extent in both noninfected and infected cells with Human Lymphotropic-T Virus type 1. Finally, oral administration of IFN-CT NPs (0.3 MIU) to CF1 mice showed detectable levels of IFNα in plasma after 1 h, whereas no IFNα was detected with a commercial formulation. These results are encouraging and open a new avenue for the administration of this biological drug in a minimally invasive, safer, and more patient-compliant way.
中文翻译:

基于壳聚糖纳米粒子口服给药干扰素-α的药物递送系统的开发
尽管干扰素-α(IFNα)在治疗某些类型的癌症和病毒感染方面具有良好的临床疗效,但由于其严重的不良反应和高剂量的肠胃外治疗方案,这种生物药物仍未得到充分利用。为了在IFNα疗法上取得突破,这项工作首次报道了用于口服给药的载有IFNα-2b的壳聚糖纳米颗粒(IFN-CT NPs)的新型纳米药物的设计和全面表征。通过离子凝胶法产生的IFN-CT NPs封装了约100%的药物,其大小为36±8 nm,ζ电位为+30 mV(动态光散射),并且呈球形(透射电子显微镜)。IFN-CT NPs的体外抗病毒活性与市售IFNα相当。值得注意的是 两种处理均在未感染和感染1型人类淋巴T病毒的细胞中以相同的程度刺激了IFN反应基因的表达。最后,对CF1小鼠口服IFN-CT NP(0.3 MIU)后,在小鼠体内可检测到IFNα水平。 1小时后血浆中没有血浆,而市售制剂中未检测到IFNα。这些结果令人鼓舞,并为以微创,更安全,更符合患者需求的方式管理这种生物药物开辟了新途径。
更新日期:2017-09-08
中文翻译:

基于壳聚糖纳米粒子口服给药干扰素-α的药物递送系统的开发
尽管干扰素-α(IFNα)在治疗某些类型的癌症和病毒感染方面具有良好的临床疗效,但由于其严重的不良反应和高剂量的肠胃外治疗方案,这种生物药物仍未得到充分利用。为了在IFNα疗法上取得突破,这项工作首次报道了用于口服给药的载有IFNα-2b的壳聚糖纳米颗粒(IFN-CT NPs)的新型纳米药物的设计和全面表征。通过离子凝胶法产生的IFN-CT NPs封装了约100%的药物,其大小为36±8 nm,ζ电位为+30 mV(动态光散射),并且呈球形(透射电子显微镜)。IFN-CT NPs的体外抗病毒活性与市售IFNα相当。值得注意的是 两种处理均在未感染和感染1型人类淋巴T病毒的细胞中以相同的程度刺激了IFN反应基因的表达。最后,对CF1小鼠口服IFN-CT NP(0.3 MIU)后,在小鼠体内可检测到IFNα水平。 1小时后血浆中没有血浆,而市售制剂中未检测到IFNα。这些结果令人鼓舞,并为以微创,更安全,更符合患者需求的方式管理这种生物药物开辟了新途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号