当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multidrug Cocrystal of Anticonvulsants: Influence of Strong Intermolecular Interactions on Physiochemical Properties
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.cgd.7b00741 Ramanpreet Kaur 1 , Katie L Cavanagh 2 , Naír Rodríguez-Hornedo 2 , Adam J Matzger 1, 3
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.cgd.7b00741 Ramanpreet Kaur 1 , Katie L Cavanagh 2 , Naír Rodríguez-Hornedo 2 , Adam J Matzger 1, 3
Affiliation
|
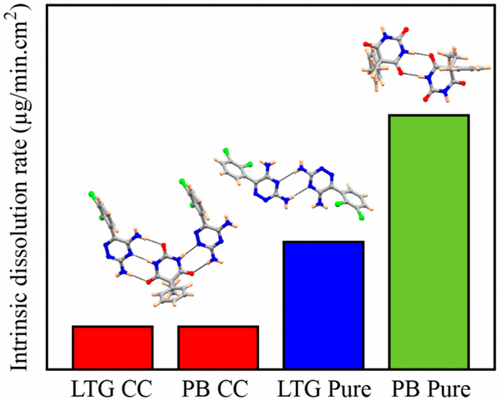
A drug–drug cocrystal of two anticonvulsants, lamotrigine and phenobarbital, is presented. In the crystal structure, molecules form heterodimers via N–H···O and N–H···N hydrogen bonding. The intrinsic dissolution rate (IDR) and solubility of the cocrystal were measured in phosphate buffer (pH 7.2) and simulated gastric fluid (without pepsin), and compared to pure APIs. Dissolution experiments found suppressed IDR of the cocrystal with rates in the order pure PB > pure LTG > cocrystal. The solubility measurements were consistent with the dissolution behavior. The presence of strong heterodimers in the cocrystal compared to weaker homodimers in the parent drugs is implicated for the reduced solubility and dissolution rate.
中文翻译:

抗惊厥药的多药共晶:强分子间相互作用对理化性质的影响
提出了两种抗惊厥药拉莫三嗪和苯巴比妥的药物-药物共晶体。在晶体结构中,分子通过N-H…O和N-H…N氢键形成异二聚体。在磷酸盐缓冲液(pH 7.2)和模拟胃液(不含胃蛋白酶)中测量共晶的固有溶出率 (IDR) 和溶解度,并与纯 API 进行比较。溶解实验发现共晶的 IDR 受到抑制,其速率按纯 PB > 纯 LTG > 共晶的顺序排列。溶解度测量结果与溶出行为一致。与母体药物中较弱的同二聚体相比,共晶中强异二聚体的存在意味着溶解度和溶出速率降低。
更新日期:2017-09-08
中文翻译:

抗惊厥药的多药共晶:强分子间相互作用对理化性质的影响
提出了两种抗惊厥药拉莫三嗪和苯巴比妥的药物-药物共晶体。在晶体结构中,分子通过N-H…O和N-H…N氢键形成异二聚体。在磷酸盐缓冲液(pH 7.2)和模拟胃液(不含胃蛋白酶)中测量共晶的固有溶出率 (IDR) 和溶解度,并与纯 API 进行比较。溶解实验发现共晶的 IDR 受到抑制,其速率按纯 PB > 纯 LTG > 共晶的顺序排列。溶解度测量结果与溶出行为一致。与母体药物中较弱的同二聚体相比,共晶中强异二聚体的存在意味着溶解度和溶出速率降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号