Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2017-09-08 , DOI: 10.1016/j.bmcl.2017.09.011 Elirosa Minniti , Jo Ann W. Byl , Laura Riccardi , Claudia Sissi , Michela Rosini , Marco De Vivo , Anna Minarini , Neil Osheroff
|
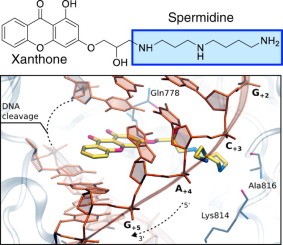
It has been proposed that xanthone derivatives with anticancer potential act as topoisomerase II inhibitors because they interfere with the ability of the enzyme to bind its ATP cofactor. In order to further characterize xanthone mechanism and generate compounds with potential as anticancer drugs, we synthesized a series of derivatives in which position 3 was substituted with different polyamine chains. As determined by DNA relaxation and decatenation assays, the resulting compounds are potent topoisomerase IIα inhibitors. Although xanthone derivatives inhibit topoisomerase IIα-catalyzed ATP hydrolysis, mechanistic studies indicate that they do not act at the ATPase site. Rather, they appear to function by blocking the ability of DNA to stimulate ATP hydrolysis. On the basis of activity, competition, and modeling studies, we propose that xanthones interact with the DNA cleavage/ligation active site of topoisomerase IIα and inhibit the catalytic activity of the enzyme by interfering with the DNA strand passage step.
中文翻译:

新型黄酮-多胺缀合物作为人类拓扑异构酶IIα的催化抑制剂
已经提出具有抗癌潜力的黄酮衍生物作为拓扑异构酶II抑制剂,因为它们干扰了该酶结合其ATP辅因子的能力。为了进一步表征x吨酮的作用机理并生成具有潜在抗癌作用的化合物,我们合成了一系列衍生物,其中第3位被不同的多胺链取代。如通过DNA弛豫和脱级测定法所确定的,所得化合物是有效的拓扑异构酶IIα抑制剂。尽管x吨酮衍生物抑制拓扑异构酶IIα催化的ATP水解,但机理研究表明它们并不作用于ATPase位点。相反,它们似乎通过阻止DNA刺激ATP水解的功能起作用。根据活动,竞争和建模研究,











































 京公网安备 11010802027423号
京公网安备 11010802027423号