Tetrahedron ( IF 2.1 ) Pub Date : 2017-09-08 , DOI: 10.1016/j.tet.2017.09.005 David A. Contreras-Cruz , Miguel A. Sánchez-Carmona , Fabio A. Vengoechea-Gómez , Daniel Peña-Ortíz , Luis D. Miranda
|
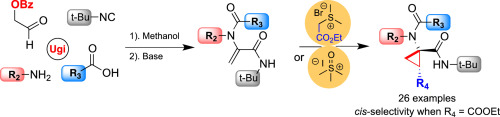
A three-step synthesis of cyclopropyl peptides is reported. The protocol involves a consecutive Ugi-4CR/elimination reaction to prepare dehydroalanines followed by a Corey-Chaykovsky cyclopropanation reaction. Peptide-like molecules that resemble some pharmacologically active compounds with a variety of substituents in the cyclopropane ring were prepared. When (2-ethoxy-2-oxoethyl) dimethyl sulfonium ylide was used the reaction exclusively gives the cis-diastereoisomer cyclopropanes in good yields from readily prepared starting materials. A collection of 26 highly substituted cyclopropyl peptides were obtained.
中文翻译:

尿素衍生的脱氢丙氨酸以多样性为导向的环丙基肽合成
报道了环丙基肽的三步合成。该方案包括连续的Ugi-4CR /消除反应以制备脱氢丙氨酸,然后进行Corey-Chaykovsky环丙烷化反应。制备类似于类药理活性化合物,在环丙烷环中具有各种取代基的肽样分子。当使用(2-乙氧基-2-氧乙基)二甲基sulf叶立德时,反应容易地由容易制备的起始原料以高收率独家得到顺式-非对映异构体环丙烷。获得了26个高度取代的环丙基肽的集合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号