当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
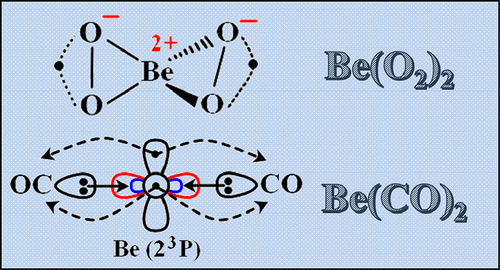
The Versatile Personality of Beryllium: Be(O2)1–2 vs Be(CO)1–2
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.jpca.7b06519 Isuru R. Ariyarathna 1 , Evangelos Miliordos 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.jpca.7b06519 Isuru R. Ariyarathna 1 , Evangelos Miliordos 1
Affiliation
|
To reveal the diverse chemistry of beryllium, we employ multireference methodologies to study the ground and several excited electronic states of the titled beryllium oxides and carbonyls. The two types of complexes serve as model systems to describe the various ways that beryllium can form chemical bonds. Be(O2), its isomer OBeO, and Be(O2)2 are ionic compounds where beryllium is best represented in its Be(II) oxidation state. On the other hand, CO induces the excitation of one or two 2s electrons of beryllium to its 2p shell. In this manner, the beryllium core (Be2+) is exposed and enables the formation of dative bonds from the lone pair of carbonyls to Be. For all of the considered electronic states, we provide accurate optimal geometries and excitation energies.
中文翻译:

铍的多功能人格:Be(O 2)1-2 vs Be(CO)1-2
为了揭示铍的不同化学性质,我们采用了多种参考方法来研究标题为铍氧化物和羰基的基态和几种激发的电子态。两种类型的配合物可作为模型系统来描述铍形成化学键的各种方式。Be(O 2),其异构体OBeO和Be(O 2)2是离子化合物,其中铍最能代表其Be(II)氧化态。另一方面,CO诱导铍的一个或两个2s电子激发到其2p壳层。铍芯(Be 2+)暴露在外,并使得能够从孤立的羰基对形成Be形成键。对于所有考虑的电子状态,我们提供准确的最佳几何形状和激发能。
更新日期:2017-09-08
中文翻译:

铍的多功能人格:Be(O 2)1-2 vs Be(CO)1-2
为了揭示铍的不同化学性质,我们采用了多种参考方法来研究标题为铍氧化物和羰基的基态和几种激发的电子态。两种类型的配合物可作为模型系统来描述铍形成化学键的各种方式。Be(O 2),其异构体OBeO和Be(O 2)2是离子化合物,其中铍最能代表其Be(II)氧化态。另一方面,CO诱导铍的一个或两个2s电子激发到其2p壳层。铍芯(Be 2+)暴露在外,并使得能够从孤立的羰基对形成Be形成键。对于所有考虑的电子状态,我们提供准确的最佳几何形状和激发能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号