当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and C–H Functionalization Chemistry of Thiazole-Semicoronenediimides (TsCDIs) and -Coronenediimides (TCDIs)
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.joc.7b01604 Qinqin Shi 1 , Eric S. Andreansky 1 , Seth R. Marder 2 , Simon B. Blakey 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.joc.7b01604 Qinqin Shi 1 , Eric S. Andreansky 1 , Seth R. Marder 2 , Simon B. Blakey 1
Affiliation
|
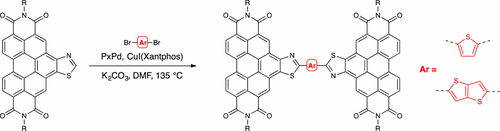
Coronenediimide (CDI) derivatives have a planar structure, a reasonably high electron affinity, and a rigid and extended delocalized π-system. Therefore, this core and variants thereof may be promising building blocks for the synthesis of electron transport materials. Herein, we have synthesized thiazole-semicoronenediimides (TsCDIs) and -coronenediimides (TCDIs) by a two-step process from a perylenediimide (PDI) precursor. Conditions for C–H arylation and heteroarylation of the thiazole moiety of this core were developed and were successfully used for the synthesis of dimer, triad, and polymeric materials. The optical and electrochemical properties of these materials and their monomers were examined as a function of side-chain modification and π-extension. With their broad optical absorption and low reduction potentials, these materials could be candidates as organic semiconductors for applications in OFETs and as nonfullerene acceptors.
中文翻译:

噻唑-半硅氧烷二酰亚胺(TsCDIs)和-Coronenediimides(TCDIs)的合成和CH官能化学
Coronenediimide(CDI)衍生物具有平面结构,相当高的电子亲和力以及刚性和扩展的离域π系统。因此,该核及其变体可能是合成电子传输材料的有前途的构建基块。在这里,我们已经从step二酰亚胺(PDI)前驱体通过两步工艺合成了噻唑-半硅氧烷二酰亚胺(TsCDIs)和-钴二酰亚胺(TCDIs)。开发了该核的噻唑部分的CH芳基化和杂芳基化的条件,并将其成功地用于二聚体,三单元组和聚合物材料的合成。检查了这些材料及其单体的光学和电化学性质,它们是侧链改性和π延伸的函数。由于其广泛的光吸收和低还原电位,
更新日期:2017-09-08
中文翻译:

噻唑-半硅氧烷二酰亚胺(TsCDIs)和-Coronenediimides(TCDIs)的合成和CH官能化学
Coronenediimide(CDI)衍生物具有平面结构,相当高的电子亲和力以及刚性和扩展的离域π系统。因此,该核及其变体可能是合成电子传输材料的有前途的构建基块。在这里,我们已经从step二酰亚胺(PDI)前驱体通过两步工艺合成了噻唑-半硅氧烷二酰亚胺(TsCDIs)和-钴二酰亚胺(TCDIs)。开发了该核的噻唑部分的CH芳基化和杂芳基化的条件,并将其成功地用于二聚体,三单元组和聚合物材料的合成。检查了这些材料及其单体的光学和电化学性质,它们是侧链改性和π延伸的函数。由于其广泛的光吸收和低还原电位,











































 京公网安备 11010802027423号
京公网安备 11010802027423号