当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereoselective Ritter-like Reaction on Cyclic Trifluoromethylated N,O-Acetals Derived from l-Tartaric Acid
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.joc.7b01814 Abdelkhalek Ben Jamaa 1 , Fabienne Grellepois 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.joc.7b01814 Abdelkhalek Ben Jamaa 1 , Fabienne Grellepois 1
Affiliation
|
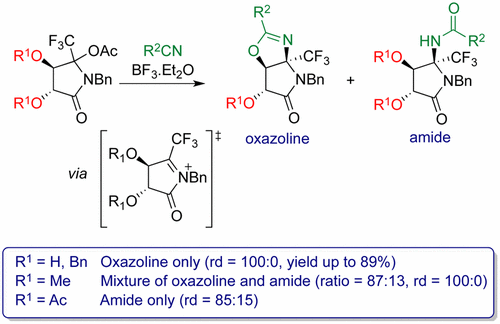
Despite the presence of the highly electron-withdrawing fluorinated substituent, cyclic α-trifluoromethylated N-acyliminium ions were successfully generated from fluorinated O-acetyl-N,O-acetal l-tartaric acid derivatives. The addition of nitriles on these intermediates occurred with high to excellent syn diastereoselectivity and led, in most cases, to oxazolines and amides as single diastereomers. The diastereoselectivity of the addition and the nature of the reaction product depend on the substituents on the hydroxyl groups of the tartaric acid scaffold. This methodology gave access to enantiopure, highly functionalized 5-(trifluoromethyl)pyrrolidin-2-one derivatives, bearing the fluorinated substituent on a tetrasubstituted carbon.
中文翻译:

l-酒石酸衍生的环状三氟甲基化N,O-缩醛的非对映选择性Ritter-like反应
尽管存在高度吸电子的氟化取代基,但还是从氟化的O-乙酰基N,O-乙缩醛1-酒石酸衍生物成功生成了环状α-三氟甲基化的N-酰基亚胺离子。在这些中间体上以高至极高的合成力添加腈非对映选择性,并在大多数情况下导致恶唑啉和酰胺为单一非对映异构体。添加的非对映选择性和反应产物的性质取决于酒石酸支架的羟基上的取代基。该方法获得了对映纯的,高度官能化的5-(三氟甲基)吡咯烷-2-酮衍生物,其在四取代的碳上带有氟化取代基。
更新日期:2017-09-08
中文翻译:

l-酒石酸衍生的环状三氟甲基化N,O-缩醛的非对映选择性Ritter-like反应
尽管存在高度吸电子的氟化取代基,但还是从氟化的O-乙酰基N,O-乙缩醛1-酒石酸衍生物成功生成了环状α-三氟甲基化的N-酰基亚胺离子。在这些中间体上以高至极高的合成力添加腈非对映选择性,并在大多数情况下导致恶唑啉和酰胺为单一非对映异构体。添加的非对映选择性和反应产物的性质取决于酒石酸支架的羟基上的取代基。该方法获得了对映纯的,高度官能化的5-(三氟甲基)吡咯烷-2-酮衍生物,其在四取代的碳上带有氟化取代基。











































 京公网安备 11010802027423号
京公网安备 11010802027423号