当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ligand Tuning in Pyridine-Alkoxide Ligated Cp*IrIII Oxidation Catalysts
Organometallics ( IF 2.8 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.organomet.7b00492 Emma V. Sackville 1 , Gabriele Kociok-Köhn 2 , Ulrich Hintermair 1
Organometallics ( IF 2.8 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.organomet.7b00492 Emma V. Sackville 1 , Gabriele Kociok-Köhn 2 , Ulrich Hintermair 1
Affiliation
|
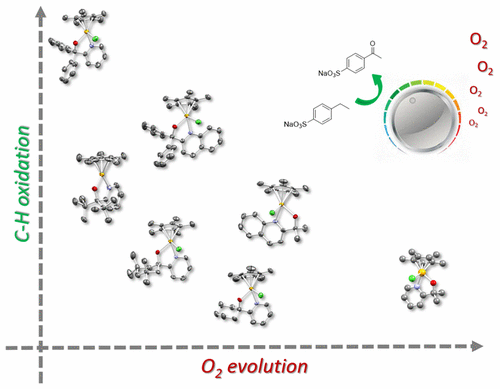
Six novel derivatives of pyridine-alkoxide ligated Cp*IrIII complexes, potent precursors for homogeneous water and C–H oxidation catalysts, have been synthesized, characterized, and analyzed spectroscopically and kinetically for ligand effects. Variation of alkoxide and pyridine substituents was found to affect their solution speciation, activation behavior, and oxidation kinetics. Application of these precursors to catalytic C–H oxidation of ethyl benzenesulfonate with aqueous sodium periodate showed that the ligand substitution pattern, solution pH, and solvent all have pronounced influences on initial rates and final conversion values. Correlation with O2 evolution profiles during C–H oxidation catalysis showed these competing reactions to occur sequentially, and demonstrates how it is possible to tune the activity and selectivity of the active species through the N^O ligand structure.
中文翻译:

吡啶-烷氧基化的Cp * Ir III氧化催化剂中的配体调节
吡啶醇盐连接的六种新型衍生物Cp * Ir III配合物(均质水和CH-H氧化催化剂的有效前体)已经合成,表征并通过光谱和动力学分析了配体效应。发现醇盐和吡啶取代基的变化会影响它们的溶液形态,活化行为和氧化动力学。这些前体在高碘酸钠水溶液催化CH-H氧化苯磺酸乙酯中的应用表明,配体的取代方式,溶液的pH值和溶剂都对初始速率和最终转化率有显着影响。与O 2相关 在C–H氧化催化过程中的演化曲线表明,这些竞争反应是相继发生的,并证明了如何通过N ^ O配体结构调节活性物种的活性和选择性。
更新日期:2017-09-08
中文翻译:

吡啶-烷氧基化的Cp * Ir III氧化催化剂中的配体调节
吡啶醇盐连接的六种新型衍生物Cp * Ir III配合物(均质水和CH-H氧化催化剂的有效前体)已经合成,表征并通过光谱和动力学分析了配体效应。发现醇盐和吡啶取代基的变化会影响它们的溶液形态,活化行为和氧化动力学。这些前体在高碘酸钠水溶液催化CH-H氧化苯磺酸乙酯中的应用表明,配体的取代方式,溶液的pH值和溶剂都对初始速率和最终转化率有显着影响。与O 2相关 在C–H氧化催化过程中的演化曲线表明,这些竞争反应是相继发生的,并证明了如何通过N ^ O配体结构调节活性物种的活性和选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号