当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multifunctional Analogs of Kynurenic Acid for the Treatment of Alzheimer’s Disease: Synthesis, Pharmacology, and Molecular Modeling Studies
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acschemneuro.7b00229 Girdhar Singh Deora 1 , Srinivas Kantham 1 , Stephen Chan 1 , Satish N. Dighe 1 , Suresh K. Veliyath 1 , Gawain McColl 2 , Marie-Odile Parat 1 , Ross P. McGeary 3 , Benjamin P. Ross 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acschemneuro.7b00229 Girdhar Singh Deora 1 , Srinivas Kantham 1 , Stephen Chan 1 , Satish N. Dighe 1 , Suresh K. Veliyath 1 , Gawain McColl 2 , Marie-Odile Parat 1 , Ross P. McGeary 3 , Benjamin P. Ross 1
Affiliation
|
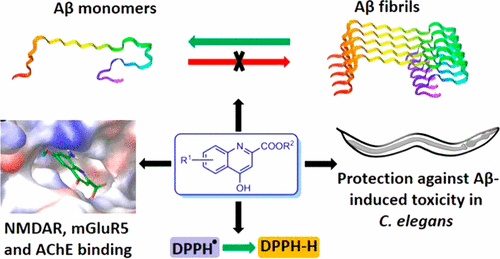
We report the synthesis and pharmacological investigation of analogs of the endogenous molecule kynurenic acid (KYNA) as multifunctional agents for the treatment of Alzheimer’s disease (AD). Synthesized KYNA analogs were tested for their N-methyl-d-aspartate (NMDA) receptor binding, mGluR5 binding and function, acetylcholinesterase (AChE) inhibition, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, interference with the amyloid β peptide (Aβ) fibrillation process, and protection against Aβ-induced toxicity in transgenic Caenorhabditis elegans strain GMC101 expressing full-length Aβ42. Molecular modeling studies were also performed to predict the binding modes of most active compounds with NMDAR, mGluR5, and Aβ42. Among the synthesized analogs, 3c, 5b, and 5c emerged as multifunctional compounds that act via multiple anti-AD mechanisms including AChE inhibition, free radical scavenging, NMDA receptor binding, mGluR5 binding, inhibition of Aβ42 fibril formation, and disassembly of preformed Aβ42 fibrils. Interestingly, 5c showed protection against Aβ42-induced toxicity in transgenic C. elegans strain GMC101. Moreover, 5b and 5c displayed high permeability in an MDR1-MDCKII cell-based model of the blood–brain barrier (BBB). Compound 3b emerged with specific activity as a micromolar AChE inhibitor, however it had low permeability in the BBB model. This study highlights the opportunities that exist to develop analogs of endogenous molecules from the kynurenine pathway for therapeutic uses.
中文翻译:

犬尿酸的多功能类似物用于治疗阿尔茨海默氏病:合成,药理学和分子模型研究
我们报告内源性分子犬尿酸(KYNA)类似物作为治疗阿尔茨海默氏病(AD)的多功能药物的类似物的合成和药理研究。合成KYNA类似物为他们测试Ñ甲基d天冬氨酸(NMDA)受体结合,mGluR5的结合和功能,乙酰胆碱酯酶抑制作用,2,2-二苯基-1-苦基苯肼(DPPH)自由基清除,干扰与淀粉样蛋白在表达全长Aβ42的转基因秀丽隐杆线虫菌株GMC101中,β肽(Aβ)的原纤化过程以及对Aβ诱导的毒性的保护作用。还进行了分子建模研究,以预测大多数活性化合物与NMDAR,mGluR5和Aβ42的结合模式。在合成的类似物中,3c,5b和5c以多功能化合物的形式出现,它们通过多种抗AD机制起作用,包括抑制AChE,清除自由基,NMDA受体结合,mGluR5结合,抑制Aβ42纤丝形成以及分解预形成的Aβ。42个原纤维。有趣的是,5c在转基因秀丽隐杆线虫菌株GMC101中显示出针对Aβ42诱导的毒性的保护作用。此外,5b和5c在基于MDR1-MDCKII细胞的血脑屏障(BBB)模型中显示出高渗透性。化合物3b作为微摩尔AChE抑制剂具有特定活性,但在BBB模型中通透性较低。这项研究强调了从犬尿氨酸途径开发内源性分子类似物用于治疗用途的机会。
更新日期:2017-09-08
中文翻译:

犬尿酸的多功能类似物用于治疗阿尔茨海默氏病:合成,药理学和分子模型研究
我们报告内源性分子犬尿酸(KYNA)类似物作为治疗阿尔茨海默氏病(AD)的多功能药物的类似物的合成和药理研究。合成KYNA类似物为他们测试Ñ甲基d天冬氨酸(NMDA)受体结合,mGluR5的结合和功能,乙酰胆碱酯酶抑制作用,2,2-二苯基-1-苦基苯肼(DPPH)自由基清除,干扰与淀粉样蛋白在表达全长Aβ42的转基因秀丽隐杆线虫菌株GMC101中,β肽(Aβ)的原纤化过程以及对Aβ诱导的毒性的保护作用。还进行了分子建模研究,以预测大多数活性化合物与NMDAR,mGluR5和Aβ42的结合模式。在合成的类似物中,3c,5b和5c以多功能化合物的形式出现,它们通过多种抗AD机制起作用,包括抑制AChE,清除自由基,NMDA受体结合,mGluR5结合,抑制Aβ42纤丝形成以及分解预形成的Aβ。42个原纤维。有趣的是,5c在转基因秀丽隐杆线虫菌株GMC101中显示出针对Aβ42诱导的毒性的保护作用。此外,5b和5c在基于MDR1-MDCKII细胞的血脑屏障(BBB)模型中显示出高渗透性。化合物3b作为微摩尔AChE抑制剂具有特定活性,但在BBB模型中通透性较低。这项研究强调了从犬尿氨酸途径开发内源性分子类似物用于治疗用途的机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号