Molecular Cell ( IF 14.5 ) Pub Date : 2017-09-07 , DOI: 10.1016/j.molcel.2017.08.004 Priyanka Sahasrabudhe , Julia Rohrberg , Maximillian M. Biebl , Daniel A. Rutz , Johannes Buchner
|
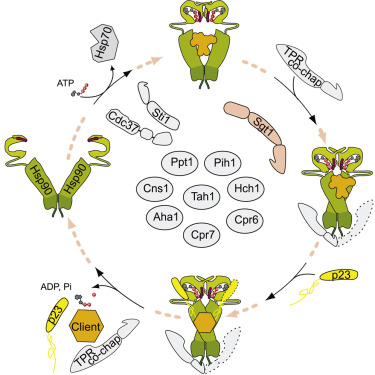
The Hsp90 system in the eukaryotic cytosol is characterized by a cohort of co-chaperones that bind to Hsp90 and affect its function. Although progress has been made regarding the underlying biochemical mechanisms, how co-chaperones influence Hsp90 client proteins in vivo has remained elusive. By investigating the effect of 12 Hsp90 co-chaperones on the activity of different client proteins in yeast, we find that deletion of co-chaperones can have a neutral or negative effect on client activity but can also lead to more active clients. Only a few co-chaperones are active on all clients studied. Closely related clients and even point mutants can depend on different co-chaperones. These effects are direct because differences in client-co-chaperone interactions can be reconstituted in vitro. Interestingly, some co-chaperones affect client conformation in vivo. Thus, co-chaperones adapt the Hsp90 cycle to the requirements of the client proteins, ensuring optimal activation.
中文翻译:

Hsp90伴侣蛋白系统的可塑性
真核细胞质中的Hsp90系统的特征是与Hsp90结合并影响其功能的辅分子伴侣。尽管在基本的生化机制方面已取得进展,但伴侣伴侣如何在体内影响Hsp90客户蛋白质仍不清楚。通过研究12种Hsp90伴侣蛋白对酵母中不同客户蛋白活性的影响,我们发现删除伴侣蛋白可对客户活性产生中性或负面影响,但也会导致更多的活跃客户。在研究的所有客户中,只有几个伴侣分子是活跃的。密切相关的客户甚至点突变体都可以依赖于不同的伴侣蛋白。这些效果是直接的,因为可以在体外重构客户-伴侣伴侣相互作用中的差异。有趣的是,一些伴侣伴侣会影响体内的客户构象。因此,陪伴分子使Hsp90循环适应客户蛋白质的需求,从而确保最佳活化。









































 京公网安备 11010802027423号
京公网安备 11010802027423号