Molecular Cell ( IF 14.5 ) Pub Date : 2017-09-07 , DOI: 10.1016/j.molcel.2017.08.007 Homa Ghalei 1 , Juliette Trepreau 1 , Jason C Collins 1 , Hari Bhaskaran 1 , Bethany S Strunk 1 , Katrin Karbstein 1
|
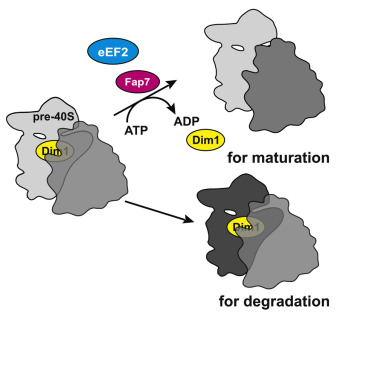
Late in their maturation, nascent small (40S) ribosomal subunits bind 60S subunits to produce 80S-like ribosomes. Because of the analogy of this translation-like cycle to actual translation, and because 80S-like ribosomes do not produce any protein, it has been suggested that this represents a quality control mechanism for subunit functionality. Here we use genetic and biochemical experiments to show that the essential ATPase Fap7 promotes formation of the rotated state, a key intermediate in translocation, thereby releasing the essential assembly factor Dim1 from pre-40S subunits. Bypassing this quality control step produces defects in reading frame maintenance. These results show how progress in the maturation cascade is linked to a test for a key functionality of 40S ribosomes: their ability to translocate the mRNA⋅tRNA pair. Furthermore, our data demonstrate for the first time that the translation-like cycle is a quality control mechanism that ensures the fidelity of the cellular ribosome pool.
中文翻译:

ATPase Fap7 测试在 40S 核糖体成熟期间进行类似易位的构象变化和释放 Dim1 的能力
在它们成熟的后期,新生的小 (40S) 核糖体亚基结合 60S 亚基产生 80S 样核糖体。由于这种翻译样循环与实际翻译的类比,并且因为 80S 样核糖体不产生任何蛋白质,有人认为这代表了亚基功能的质量控制机制。在这里,我们使用遗传和生化实验来证明必需的 ATPase Fap7 促进旋转状态的形成,旋转状态是易位的关键中间体,从而从 40S 前亚基释放必需的装配因子 Dim1。绕过这个质量控制步骤会在阅读框架维护中产生缺陷。这些结果显示了成熟级联反应的进展如何与 40S 核糖体的关键功能测试相关联:它们转移 mRNA⋅tRNA 对的能力。此外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号