当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational Entropy from Slowly Relaxing Local Structure Analysis of 15N–H Relaxation in Proteins: Application to Pheromone Binding to MUP-I in the 283–308 K Temperature Range
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.jpcb.7b06049 Lukáš Žídek 1, 2 , Eva Meirovitch 3
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.jpcb.7b06049 Lukáš Žídek 1, 2 , Eva Meirovitch 3
Affiliation
|
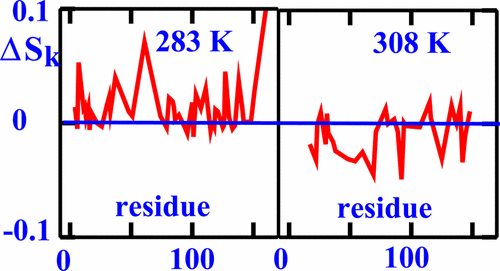
The slowly relaxing local structure (SRLS) approach is applied to 15N–H relaxation from the major urinary protein I (MUP-I), and its complex with pheromone 2-sec-butyl-4,5-dihydrothiazol. The objective is to elucidate dynamics, and binding-induced changes in conformational entropy. Experimental data acquired previously in the 283–308 K temperature range are used. The N–H bond is found to reorient globally with correlation time, τ1,0, and locally with correlation time, τ2,0, where τ1,0 ≫ τ2,0. The local motion is restricted by the potential u = −c02D002, where D002 is the Wigner rotation matrix element for L = 2, K = 0, and c02 evaluates the strength of the potential. u yields straightforwardly the order parameter, ⟨D002⟩, and the conformational entropy, Sk, both given by Peq = exp(−u). The deviation of the local ordering/local diffusion axis from the N–H bond, given by the angle β, is also determined. We find that c02 ≅ 18 ± 4 and τ2,0 = 0–170 ps for ligand-free MUP-I, whereas c02 ≅ 15 ± 4 and τ2,0 = 20–270 ps for ligand-bound MUP-I. β is in the 0–10° range. c02 and τ2,0 decrease, whereas β increases, when the temperature is increased from 283 to 308 K. Thus, SRLS provides physically well-defined structure-related (c02 and ⟨D002⟩), motion-related (τ2,0), geometry-related (β), and binding-related (Sk) local parameters, and their temperature-dependences. Intriguingly, upon pheromone binding the conformational entropy of MUP-I decreases at high temperature and increases at low temperature. The very same experimental data were analyzed previously with the model-free (MF) method which yielded “global” (in this context, “relating to the entire 283–308 K range”) amplitude (S2) and rate (τe) of the local motion, and a phenomenological exchange term (Rex). S2 is found to decrease (implying implicitly “global” increase in Sk) upon pheromone binding.
中文翻译:

蛋白质在15 N–H弛豫中缓慢弛豫的局部结构分析引起的构象熵:在283–308 K温度范围内信息素与MUP-I结合的应用
缓慢松弛局部结构(SRLS)方法可用于从主要尿蛋白I(MUP-1)及其与信息素2 - sec -butyl-4,5-dihydrothiazol的复合体中释放15 N–H 。目的是阐明动力学和结合诱导的构象熵变化。使用先前在283–308 K温度范围内获取的实验数据。的N-H键被发现重新定向与全局相关时间,τ 1,0,并在本地与相关时间,τ 2,0,其中τ 1,0 »τ 2,0。局部运动受电势u = − c 0 2 D 00 2的限制,其中D 00 2是维格纳旋转矩阵元素,其中L = 2,K = 0,并且c 0 2评估电势的强度。ü直截了当产生的有序参数,⟨ d 00 2 ⟩,和构象熵,小号ķ,由两个给定P当量= EXP( - Ú)。还确定了局部有序/局部扩散轴与N–H键的偏离,由角度β给出。我们发现,c ^ 0 2 ≅18±4和τ 2,0 = 0 -170个皮秒为无配体的MUP-I,而c ^ 0 2 ≅15±4和τ 2,0 = 20 - 270皮秒为配体-结合的MUP-I。β在0 – 10°的范围内。c ^ 0 2和τ 2,0降低,而β的增加,当温度从283增加至308 K.因此,SRLS提供物理良好定义的结构相关的(C ^ 0 2和⟨ d 00 2 ⟩),MOTION-相关(τ2,0),几何相关(β)和绑定相关(S k)局部参数及其温度依赖性。有趣的是,信息素结合后,MUP-1的构象熵在高温下降低,而在低温下升高。非常相同的实验数据(“与整个283-308 K范围之外”在此上下文中,)振幅(用其产生“全局”的无模型(MF)方法之前分析的小号2)和率(τ ë)局部运动和现象交换术语(R ex)。发现在信息素结合后,S 2降低(暗示S k隐式“整体”增加)。
更新日期:2017-09-08
中文翻译:

蛋白质在15 N–H弛豫中缓慢弛豫的局部结构分析引起的构象熵:在283–308 K温度范围内信息素与MUP-I结合的应用
缓慢松弛局部结构(SRLS)方法可用于从主要尿蛋白I(MUP-1)及其与信息素2 - sec -butyl-4,5-dihydrothiazol的复合体中释放15 N–H 。目的是阐明动力学和结合诱导的构象熵变化。使用先前在283–308 K温度范围内获取的实验数据。的N-H键被发现重新定向与全局相关时间,τ 1,0,并在本地与相关时间,τ 2,0,其中τ 1,0 »τ 2,0。局部运动受电势u = − c 0 2 D 00 2的限制,其中D 00 2是维格纳旋转矩阵元素,其中L = 2,K = 0,并且c 0 2评估电势的强度。ü直截了当产生的有序参数,⟨ d 00 2 ⟩,和构象熵,小号ķ,由两个给定P当量= EXP( - Ú)。还确定了局部有序/局部扩散轴与N–H键的偏离,由角度β给出。我们发现,c ^ 0 2 ≅18±4和τ 2,0 = 0 -170个皮秒为无配体的MUP-I,而c ^ 0 2 ≅15±4和τ 2,0 = 20 - 270皮秒为配体-结合的MUP-I。β在0 – 10°的范围内。c ^ 0 2和τ 2,0降低,而β的增加,当温度从283增加至308 K.因此,SRLS提供物理良好定义的结构相关的(C ^ 0 2和⟨ d 00 2 ⟩),MOTION-相关(τ2,0),几何相关(β)和绑定相关(S k)局部参数及其温度依赖性。有趣的是,信息素结合后,MUP-1的构象熵在高温下降低,而在低温下升高。非常相同的实验数据(“与整个283-308 K范围之外”在此上下文中,)振幅(用其产生“全局”的无模型(MF)方法之前分析的小号2)和率(τ ë)局部运动和现象交换术语(R ex)。发现在信息素结合后,S 2降低(暗示S k隐式“整体”增加)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号