当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Competition between HO2 and H2O2 Reactions with CH2OO/anti-CH3CHOO in the Oligomer Formation: A Theoretical Perspective
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.jpca.7b05951 Long Chen 1, 2 , Yu Huang 1, 2 , Yonggang Xue 1, 2 , Junji Cao 1, 2 , Wenliang Wang 3
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.jpca.7b05951 Long Chen 1, 2 , Yu Huang 1, 2 , Yonggang Xue 1, 2 , Junji Cao 1, 2 , Wenliang Wang 3
Affiliation
|
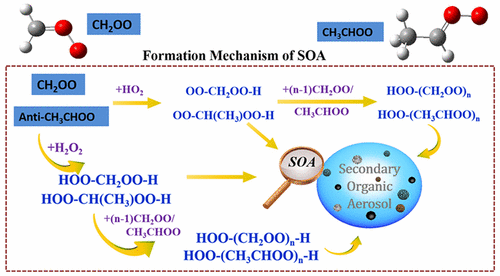
Understanding Criegee chemistry has become one of the central topics in atmospheric studies recently. Ozonolysis of unsaturated hydrocarbons is believed to be an important pathway of secondary organic aerosol (SOA). However, the SOA formation mechanisms via Criegee chemistry are still poorly understood. Here, we systematically study the competition between HO2 and H2O2 reactions with CH2OO/anti-CH3CHOO in the oligomer formations. The calculated results show that oligomers having Criegee intermediates as the chain units are produced by the sequential addition of Criegee intermediates (CIs) to HO2 and H2O2 molecules. The addition reactions are predicted to be strongly exothermic, and the apparent activation barriers are estimated to be negative, suggesting that these reactions are feasible both thermochemically and dynamically. Compared to the barriers of 4CH2OO + HO2 and 4CH2OO + H2O2 reactions, it can be found that the first two CH2OO addition reactions in the former case are favored, while the last two CH2OO addition reactions in the latter case are preferable. A similar conclusion is also obtained from those of the 4anti-CH3CHOO + HO2/H2O2 systems. The mechanistic insights can motivate future experimental studies of the effect of longer-chain CIs on the formation of SOA, which plays an important role on air quality and climate change.
中文翻译:

CH 2 OO /反CH 3 CHOO在低聚物形成中HO 2和H 2 O 2反应之间的竞争:理论观点
近年来,了解Criegee化学已成为大气研究的中心主题之一。不饱和烃的臭氧分解被认为是二次有机气溶胶(SOA)的重要途径。但是,通过Criegee化学方法形成SOA的机制仍然知之甚少。在这里,我们系统地研究了低聚物形成中HO 2和H 2 O 2与CH 2 OO /抗-CH 3 CHOO之间的竞争。计算结果表明,通过将Criegee中间体(CIs)依次添加到HO 2和H 2 O 2中,可以生产以Cegeege中间体为链单元的低聚物。分子。预测加成反应是强烈放热的,并且表观活化势垒被认为是负的,表明这些反应在热化学和动态上都是可行的。与4CH 2 OO + HO 2和4CH 2 OO + H 2 O 2反应的势垒相比,可以发现在前一种情况下前两个CH 2 OO加成反应是有利的,而最后两个CH 2 OO加成反应是有利的在后一种情况下反应是优选的。从4种抗-CH 3 CHOO + HO 2 / H 2 O 2的结果也得出类似的结论。系统。机械学的见解可以激发未来关于长链CI对SOA形成的影响的实验研究,而SOA在空气质量和气候变化中起着重要作用。
更新日期:2017-09-08
中文翻译:

CH 2 OO /反CH 3 CHOO在低聚物形成中HO 2和H 2 O 2反应之间的竞争:理论观点
近年来,了解Criegee化学已成为大气研究的中心主题之一。不饱和烃的臭氧分解被认为是二次有机气溶胶(SOA)的重要途径。但是,通过Criegee化学方法形成SOA的机制仍然知之甚少。在这里,我们系统地研究了低聚物形成中HO 2和H 2 O 2与CH 2 OO /抗-CH 3 CHOO之间的竞争。计算结果表明,通过将Criegee中间体(CIs)依次添加到HO 2和H 2 O 2中,可以生产以Cegeege中间体为链单元的低聚物。分子。预测加成反应是强烈放热的,并且表观活化势垒被认为是负的,表明这些反应在热化学和动态上都是可行的。与4CH 2 OO + HO 2和4CH 2 OO + H 2 O 2反应的势垒相比,可以发现在前一种情况下前两个CH 2 OO加成反应是有利的,而最后两个CH 2 OO加成反应是有利的在后一种情况下反应是优选的。从4种抗-CH 3 CHOO + HO 2 / H 2 O 2的结果也得出类似的结论。系统。机械学的见解可以激发未来关于长链CI对SOA形成的影响的实验研究,而SOA在空气质量和气候变化中起着重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号