当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of Intramolecular Charge Transfer and Nuclear Quantum Effects on Intramolecular Hydrogen Bonds in Azopyrimidines
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.joc.7b01810 Kateřina Bártová 1, 2 , Lucie Čechová 1, 3 , Eliška Procházková 1 , Ondřej Socha 1 , Zlatko Janeba 1 , Martin Dračínský 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.joc.7b01810 Kateřina Bártová 1, 2 , Lucie Čechová 1, 3 , Eliška Procházková 1 , Ondřej Socha 1 , Zlatko Janeba 1 , Martin Dračínský 1
Affiliation
|
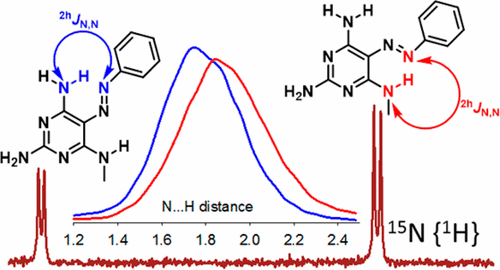
Intramolecular hydrogen bonds (IMHBs) in 5-azopyrimidines are investigated by NMR spectroscopy and DFT computations that involve nuclear quantum effects. A series of substituted 5-phenylazopyrimidines with one or two hydrogen bond donors able to form IMHBs with the azo group is prepared by azo coupling. The barrier of interconversion between two rotamers of the compounds with two possible IMHBs is determined by variable temperature NMR spectroscopy and it is demonstrated that the barrier is significantly affected by intramolecular charge transfer. Through-hydrogen-bond scalar coupling is investigated in 15N labeled compounds and the stability of the IMHBs is correlated with experimental NMR parameters and rationalized by path integral molecular dynamics simulations that involve nuclear quantum effects. Detailed information on the hydrogen bond geometry upon hydrogen-to-deuterium isotope exchange is obtained from a comparison of experimental and calculated NMR data.
中文翻译:

分子内电荷转移和核量子效应对偶氮嘧啶中分子内氢键的影响
通过核磁共振波谱法和涉及核量子效应的DFT计算研究了5-偶氮嘧啶中的分子内氢键(IMHBs)。通过偶氮偶合制备一系列具有一个或两个能够与偶氮基形成IMHB的氢键供体的取代的5-苯基偶氮嘧啶。化合物的两个旋转异构体与两个可能的IMHBs之间的互变壁垒是通过可变温度NMR光谱法确定的,证明了该壁垒受到分子内电荷转移的显着影响。通过氢键的标量耦合研究在15N标记的化合物和IMHBs的稳定性与实验NMR参数相关联,并通过涉及核量子效应的路径积分分子动力学模拟得以合理化。通过比较实验数据和计算得出的NMR数据,可以获得氢-氘同位素交换时氢键几何结构的详细信息。
更新日期:2017-09-08
中文翻译:

分子内电荷转移和核量子效应对偶氮嘧啶中分子内氢键的影响
通过核磁共振波谱法和涉及核量子效应的DFT计算研究了5-偶氮嘧啶中的分子内氢键(IMHBs)。通过偶氮偶合制备一系列具有一个或两个能够与偶氮基形成IMHB的氢键供体的取代的5-苯基偶氮嘧啶。化合物的两个旋转异构体与两个可能的IMHBs之间的互变壁垒是通过可变温度NMR光谱法确定的,证明了该壁垒受到分子内电荷转移的显着影响。通过氢键的标量耦合研究在15N标记的化合物和IMHBs的稳定性与实验NMR参数相关联,并通过涉及核量子效应的路径积分分子动力学模拟得以合理化。通过比较实验数据和计算得出的NMR数据,可以获得氢-氘同位素交换时氢键几何结构的详细信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号