Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of (±)-Allocolchicine and Its Analogues Using Co-Catalyzed Alkyne [2 + 2 + 2]-Cyclotrimerization
ACS Omega ( IF 3.7 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acsomega.7b00980 Dinesh J. Paymode 1, 2 , Chepuri V. Ramana 1, 2
ACS Omega ( IF 3.7 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acsomega.7b00980 Dinesh J. Paymode 1, 2 , Chepuri V. Ramana 1, 2
Affiliation
|
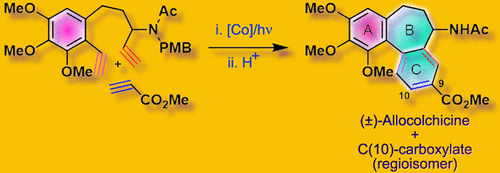
The total synthesis of (±)-allocolchicine has been completed by employing cobalt-catalyzed alkyne [2 + 2 + 2]-cyclotrimerization as the key reaction. The essential diyne has been synthesized from easily available 3,4,5-trimethoxybenzaldehyde following simple chemical transformations. In general, the cycloaddition gave a mixture of C(9) and C(10) isomers thus allowing the synthesis of both allocolchicine and its C(10)-carboxylate. Because this cycloaddition was employed at the penultimate stage, it allowed the synthesis of various analogues having the diverse functionality at C(9) and/or C(10) of ring C.
中文翻译:

共催化炔[2 + 2 + 2]-环三聚全合成(±)-别甲秋水仙碱及其类似物
通过使用钴催化的炔烃[2 + 2 + 2]-环三聚反应作为关键反应,完成了(±)-二十碳五环素的总合成。通过简单的化学转化,由容易获得的3,4,5-三甲氧基苯甲醛合成了必需的二炔。通常,通过环加成反应可得到C(9)和C(10)异构体的混合物,因此可以合成金属酚和其C(10)-羧酸盐。由于该环加成反应是在倒数第二个阶段进行的,因此它可以合成在环C的C(9)和/或C(10)处具有不同功能的各种类似物。
更新日期:2017-09-08
中文翻译:

共催化炔[2 + 2 + 2]-环三聚全合成(±)-别甲秋水仙碱及其类似物
通过使用钴催化的炔烃[2 + 2 + 2]-环三聚反应作为关键反应,完成了(±)-二十碳五环素的总合成。通过简单的化学转化,由容易获得的3,4,5-三甲氧基苯甲醛合成了必需的二炔。通常,通过环加成反应可得到C(9)和C(10)异构体的混合物,因此可以合成金属酚和其C(10)-羧酸盐。由于该环加成反应是在倒数第二个阶段进行的,因此它可以合成在环C的C(9)和/或C(10)处具有不同功能的各种类似物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号