当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
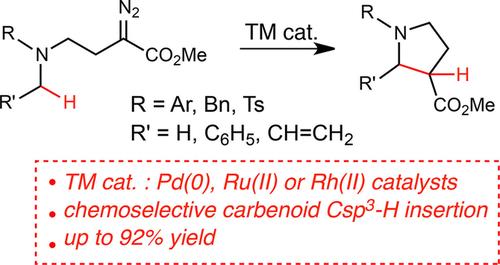
Transition Metal-Catalysed Intramolecular Carbenoid C−H Insertion for Pyrrolidine Formation by Decomposition of α-Diazoesters
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2017-09-08 03:06:01 , DOI: 10.1002/adsc.201700840 Daniel Solé 1 , Arianna Amenta 1 , Francesco Mariani 1 , M.-Lluïsa Bennasar 1 , Israel Fernández 2
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2017-09-08 03:06:01 , DOI: 10.1002/adsc.201700840 Daniel Solé 1 , Arianna Amenta 1 , Francesco Mariani 1 , M.-Lluïsa Bennasar 1 , Israel Fernández 2
Affiliation
|
The use of Pd-, Rh(II)- and Ru(II)-based catalysts has been explored in the transition metal-catalysed intramolecular carbenoid C−H insertion of α-diazoesters leading to pyrrolidines. Although the outcome of the reaction was highly substrate-dependent, in general, it was possible to control the chemoselectivity of the process towards pyrrolidines by adequate catalyst selection. The Pd(0)-catalysts were as efficient as [Rh(Ph3CCO2)2]2 in promoting the C(sp3)−H insertion of ortho-substituted anilines. In contrast, for anilines bearing meta- and para-substituents, the Rh(II)-catalyst provided the best chemoselectivities and reaction yields. On the other hand, [Ru(p-cymene)Cl2]2 was the most efficient catalyst for the insertion reaction of the N-benzyl-N-phenyl and N,N-dibenzyl α-diazoesters, while the C(sp3)−H insertion of the N-benzylsulfonamide substrate was only promoted by [Rh(Ph3CCO2)2]2. According to density functional theory (DFT) calculations, the mechanism involved in the Pd(0)- and Ru(II)-catalysed C(sp3)−H insertions differs considerably from that typically proposed for the Rh(II)-catalysed transformation. Whereas the Pd(0)-catalysed reaction involves a Pd-mediated 1,5-H migration from the C(sp3)−H bond to the carbenoid carbon atom leading to the formal oxidation of the transition metal, a Ru(II)-promoted Mannich type reaction involving a zwitterionic intermediate seems to be operative in the Ru(II)-catalysed transformation.
中文翻译:

过渡金属催化的分子内类胡萝卜素CH插入,通过α-重氮酯的分解形成吡咯烷
在过渡金属催化的分子内类固醇CH-H插入导致吡咯烷的α-二重氮中探索了基于Pd,Rh(II)和Ru(II)的催化剂的使用。尽管反应的结果高度依赖于底物,但是通常可以通过适当的催化剂选择来控制该方法对吡咯烷的化学选择性。在促进邻位取代苯胺的C(sp 3)-H插入过程中,Pd(0)催化剂与[Rh(Ph 3 CCO 2)2 ] 2一样有效。相反,对于带有meta-和para的苯胺-取代基,Rh(II)催化剂具有最佳的化学选择性和反应产率。另一方面,[Ru(p- Cymene)Cl 2 ] 2是N-苄基-N-苯基与N,N-二苄基α-二重氮酸酯插入反应的最有效催化剂,而C(sp 3仅通过[Rh(Ph 3 CCO 2)2 ] 2可以促进N-苄基磺酰胺底物的)-H插入。根据密度泛函理论(DFT)计算,Pd(0)和Ru(II)催化的C(sp3)-H插入与Rh(II)催化转化通常建议的插入有很大不同。Pd(0)催化的反应涉及Pd介导的1,5-H从C(sp 3)-H键迁移至类胡萝卜素碳原子,从而导致过渡金属Ru(II)的形式氧化两性离子中间体的苯丙胺促进的曼尼希型反应似乎在Ru(II)催化的转化中起作用。
更新日期:2017-09-08
中文翻译:

过渡金属催化的分子内类胡萝卜素CH插入,通过α-重氮酯的分解形成吡咯烷
在过渡金属催化的分子内类固醇CH-H插入导致吡咯烷的α-二重氮中探索了基于Pd,Rh(II)和Ru(II)的催化剂的使用。尽管反应的结果高度依赖于底物,但是通常可以通过适当的催化剂选择来控制该方法对吡咯烷的化学选择性。在促进邻位取代苯胺的C(sp 3)-H插入过程中,Pd(0)催化剂与[Rh(Ph 3 CCO 2)2 ] 2一样有效。相反,对于带有meta-和para的苯胺-取代基,Rh(II)催化剂具有最佳的化学选择性和反应产率。另一方面,[Ru(p- Cymene)Cl 2 ] 2是N-苄基-N-苯基与N,N-二苄基α-二重氮酸酯插入反应的最有效催化剂,而C(sp 3仅通过[Rh(Ph 3 CCO 2)2 ] 2可以促进N-苄基磺酰胺底物的)-H插入。根据密度泛函理论(DFT)计算,Pd(0)和Ru(II)催化的C(sp3)-H插入与Rh(II)催化转化通常建议的插入有很大不同。Pd(0)催化的反应涉及Pd介导的1,5-H从C(sp 3)-H键迁移至类胡萝卜素碳原子,从而导致过渡金属Ru(II)的形式氧化两性离子中间体的苯丙胺促进的曼尼希型反应似乎在Ru(II)催化的转化中起作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号