Journal of Controlled Release ( IF 10.5 ) Pub Date : 2017-09-08 , DOI: 10.1016/j.jconrel.2017.09.011 Irina Cherniakov , Dvora Izgelov , Dinorah Barasch , Elyad Davidson , Abraham J. Domb , Amnon Hoffman
|
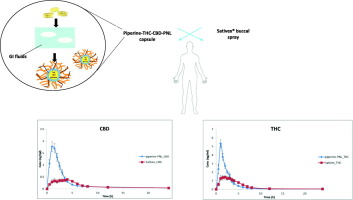
Nowadays, therapeutic indications for cannabinoids, specifically Δ9-tetrahydrocannabinol (THC) and Cannabidiol (CBD) are widening. However, the oral consumption of the molecules is very limited due to their highly lipophilic nature that leads to poor solubility at the aqueous environment. Additionally, THC and CBD are prone to extensive first pass mechanisms. These absorption obstacles render the molecules with low and variable oral bioavailability. To overcome these limitations we designed and developed the advanced pro-nanolipospheres (PNL) formulation. The PNL delivery system is comprised of a medium chain triglyceride, surfactants, a co-solvent and the unique addition of a natural absorption enhancer: piperine. Piperine was selected due to its distinctive inhibitory properties affecting both Phase I and Phase II metabolism. This constellation self emulsifies into nano particles that entrap the cannabinoids and the piperine in their core and thus improve their solubility while piperine and the other PNL excipients inhibit their intestinal metabolism. Another clear advantage of the formulation is that its composition of materials is approved for human consumption. The safe nature of the excipients enabled their direct evaluation in humans. In order to evaluate the pharmacokinetic profile of the THC-CBD-piperine-PNL formulation, a two-way crossover, single administration clinical study was conducted. The trial comprised of 9 healthy volunteers under fasted conditions. Each subject received a THC-CBD (10.8 mg, 10 mg respectively) piperine (20 mg)-PNL filled capsule and an equivalent dose of the oromucosal spray Sativex® with a washout period in between treatments.
Single oral administration of the piperine-PNL formulation resulted in a 3-fold increase in Cmax and a 1.5-fold increase in AUC for THC when compared to Sativex®. For CBD, a 4-fold increase in Cmax and a 2.2-fold increase in AUC was observed. These findings demonstrate the potential this formulation has in serving as a standardized oral cannabinoid formulation. Moreover, the concept of improving oral bioavailability described here, can pave the way for other potential lipophilic active compounds requiring enhancement of their oral bioavailability.
中文翻译:

胡椒碱原纳米脂质体作为大麻素的新型口服给药系统:与口腔喷雾剂相比,健康志愿者的药代动力学评估
如今,对大麻素治疗适应症,特别是Δ 9-四氢大麻酚(THC)和大麻二酚(CBD)正在扩大。然而,由于分子的高度亲脂性,导致其在水性环境中的溶解性差,因此它们的口服消耗非常有限。此外,THC和CBD倾向于广泛的首过机制。这些吸收障碍使分子具有较低且可变的口服生物利用度。为了克服这些限制,我们设计并开发了先进的纳米脂质体原(PNL)配方。PNL输送系统由中链甘油三酸酯,表面活性剂,助溶剂和独特的天然吸收促进剂:胡椒碱组成。选择胡椒碱是因为其独特的抑制特性会影响第一阶段和第二阶段的代谢。这种星座自我乳化成纳米颗粒,将大麻素和胡椒碱包裹在其核心中,从而提高了它们的溶解度,而胡椒碱和其他PNL赋形剂则抑制了它们的肠道代谢。该制剂的另一个明显的优点是其材料组成被批准用于人类消费。赋形剂的安全性使其能够在人体中进行直接评估。为了评估THC-CBD-胡椒碱-PNL制剂的药代动力学特征,进行了双向交叉,单次给药的临床研究。该试验由9名健康禁食的健康志愿者组成。每个受试者接受THC-CBD(分别为10.8 mg,10 mg)胡椒碱(20 mg)-PNL填充的胶囊和等剂量的口腔粘膜喷雾剂Sativex®,且在两次治疗之间要有冲洗期。
与Sativex®相比,单次口服胡椒碱-PNL制剂可使THC的Cmax增加3倍,AUC增加1.5倍。对于CBD,观察到Cmax增加4倍,AUC增加2.2倍。这些发现证明该制剂具有用作标准化口服大麻素制剂的潜力。而且,本文所述的改善口服生物利用度的概念可以为需要提高其口服生物利用度的其他潜在的亲脂性活性化合物铺平道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号