当前位置:
X-MOL 学术
›
Cell Stem Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
MicroRNAs Induce a Permissive Chromatin Environment that Enables Neuronal Subtype-Specific Reprogramming of Adult Human Fibroblasts.
Cell Stem Cell ( IF 19.8 ) Pub Date : 2017-09-07 , DOI: 10.1016/j.stem.2017.08.002 Daniel G. Abernathy , Woo Kyung Kim , Matthew J. McCoy , Allison M. Lake , Rebecca Ouwenga , Seong Won Lee , Xiaoyun Xing , Daofeng Li , Hyung Joo Lee , Robert O. Heuckeroth , Joseph D. Dougherty , Ting Wang , Andrew S. Yoo
Cell Stem Cell ( IF 19.8 ) Pub Date : 2017-09-07 , DOI: 10.1016/j.stem.2017.08.002 Daniel G. Abernathy , Woo Kyung Kim , Matthew J. McCoy , Allison M. Lake , Rebecca Ouwenga , Seong Won Lee , Xiaoyun Xing , Daofeng Li , Hyung Joo Lee , Robert O. Heuckeroth , Joseph D. Dougherty , Ting Wang , Andrew S. Yoo
|
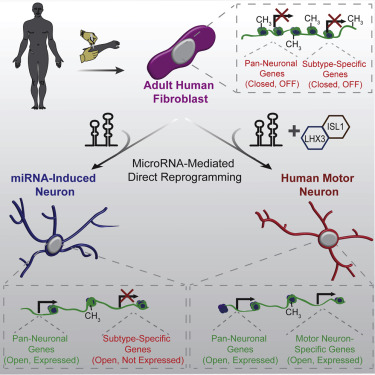
Directed reprogramming of human fibroblasts into fully differentiated neurons requires massive changes in epigenetic and transcriptional states. Induction of a chromatin environment permissive for acquiring neuronal subtype identity is therefore a major barrier to fate conversion. Here we show that the brain-enriched miRNAs miR-9/9∗ and miR-124 (miR-9/9∗-124) trigger reconfiguration of chromatin accessibility, DNA methylation, and mRNA expression to induce a default neuronal state. miR-9/9∗-124-induced neurons (miNs) are functionally excitable and uncommitted toward specific subtypes but possess open chromatin at neuronal subtype-specific loci, suggesting that such identity can be imparted by additional lineage-specific transcription factors. Consistently, we show that ISL1 and LHX3 selectively drive conversion to a highly homogeneous population of human spinal cord motor neurons. This study shows that modular synergism between miRNAs and neuronal subtype-specific transcription factors can drive lineage-specific neuronal reprogramming, providing a general platform for high-efficiency generation of distinct subtypes of human neurons.
中文翻译:

MicroRNA诱导了允许人类成纤维细胞的神经元亚型特异性重编程的染色质环境。
将人类成纤维细胞定向重编程为完全分化的神经元需要表观遗传和转录状态发生巨大变化。因此,诱导获得神经元亚型同一性的染色质环境的诱导是命运转换的主要障碍。在这里,我们显示大脑富集的miRNA miR-9 / 9 *和miR-124(miR-9 / 9 * -124)触发了染色质可访问性,DNA甲基化和mRNA表达的重新配置,以诱导默认的神经元状态。miR-9 / 9 *-124诱导的神经元(miNs)在功能上是兴奋性的,并且不致力于特定亚型,但是在神经元亚型特异性基因座上具有开放的染色质,这表明可以通过其他谱系特异性转录因子赋予这种同一性。一致地,我们表明ISL1和LHX3选择性地驱动转换为高度均匀的人类脊髓运动神经元群体。这项研究表明,miRNA与神经元亚型特异性转录因子之间的模块化协同作用可以驱动谱系特异性神经元重编程,从而为高效生成人类神经元不同亚型提供了一个通用平台。
更新日期:2017-09-07
中文翻译:

MicroRNA诱导了允许人类成纤维细胞的神经元亚型特异性重编程的染色质环境。
将人类成纤维细胞定向重编程为完全分化的神经元需要表观遗传和转录状态发生巨大变化。因此,诱导获得神经元亚型同一性的染色质环境的诱导是命运转换的主要障碍。在这里,我们显示大脑富集的miRNA miR-9 / 9 *和miR-124(miR-9 / 9 * -124)触发了染色质可访问性,DNA甲基化和mRNA表达的重新配置,以诱导默认的神经元状态。miR-9 / 9 *-124诱导的神经元(miNs)在功能上是兴奋性的,并且不致力于特定亚型,但是在神经元亚型特异性基因座上具有开放的染色质,这表明可以通过其他谱系特异性转录因子赋予这种同一性。一致地,我们表明ISL1和LHX3选择性地驱动转换为高度均匀的人类脊髓运动神经元群体。这项研究表明,miRNA与神经元亚型特异性转录因子之间的模块化协同作用可以驱动谱系特异性神经元重编程,从而为高效生成人类神经元不同亚型提供了一个通用平台。









































 京公网安备 11010802027423号
京公网安备 11010802027423号