Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lis1 Has Two Opposing Modes of Regulating Cytoplasmic Dynein.
Cell ( IF 45.5 ) Pub Date : 2017-Sep-07 , DOI: 10.1016/j.cell.2017.08.037 Morgan E. DeSantis , Michael A. Cianfrocco , Zaw Min Htet , Phuoc Tien Tran , Samara L. Reck-Peterson , Andres E. Leschziner
Cell ( IF 45.5 ) Pub Date : 2017-Sep-07 , DOI: 10.1016/j.cell.2017.08.037 Morgan E. DeSantis , Michael A. Cianfrocco , Zaw Min Htet , Phuoc Tien Tran , Samara L. Reck-Peterson , Andres E. Leschziner
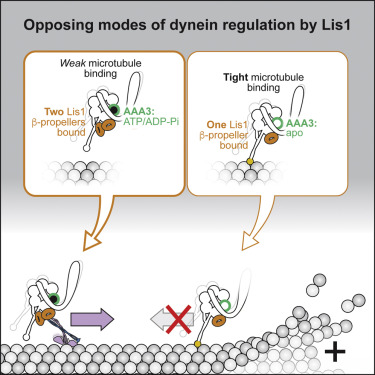
|
Regulation is central to the functional versatility of cytoplasmic dynein, a motor involved in intracellular transport, cell division, and neurodevelopment. Previous work established that Lis1, a conserved regulator of dynein, binds to its motor domain and induces a tight microtubule-binding state in dynein. The work we present here-a combination of biochemistry, single-molecule assays, and cryoelectron microscopy-led to the surprising discovery that Lis1 has two opposing modes of regulating dynein, being capable of inducing both low and high affinity for the microtubule. We show that these opposing modes depend on the stoichiometry of Lis1 binding to dynein and that this stoichiometry is regulated by the nucleotide state of dynein's AAA3 domain. The low-affinity state requires Lis1 to also bind to dynein at a novel conserved site, mutation of which disrupts Lis1's function in vivo. We propose a new model for the regulation of dynein by Lis1.
中文翻译:

Lis1有两种相反的调节细胞质动力蛋白的模式。
调节对于细胞质动力蛋白的功能多样性至关重要,动力蛋白参与细胞内运输,细胞分裂和神经发育。先前的工作证实,动力蛋白的保守调节剂Lis1与其动力结构域结合,并在动力蛋白中诱导出紧密的微管结合状态。我们在这里提出的工作-生物化学,单分子测定和冷冻电子显微镜的结合-导致令人惊讶的发现,即Lis1具有两种相反的调节动力蛋白的模式,能够诱导对微管的低亲和力和高亲和力。我们显示这些相反的模式取决于Lis1与达因结合的化学计量,并且该化学计量受达因AAA3结构域的核苷酸状态调控。低亲和力状态要求Lis1还必须在新的保守位点上与动力蛋白结合,突变会破坏Lis1的体内功能。我们提出了一种新的Lis1调控动力蛋白的模型。
更新日期:2017-09-07
中文翻译:

Lis1有两种相反的调节细胞质动力蛋白的模式。
调节对于细胞质动力蛋白的功能多样性至关重要,动力蛋白参与细胞内运输,细胞分裂和神经发育。先前的工作证实,动力蛋白的保守调节剂Lis1与其动力结构域结合,并在动力蛋白中诱导出紧密的微管结合状态。我们在这里提出的工作-生物化学,单分子测定和冷冻电子显微镜的结合-导致令人惊讶的发现,即Lis1具有两种相反的调节动力蛋白的模式,能够诱导对微管的低亲和力和高亲和力。我们显示这些相反的模式取决于Lis1与达因结合的化学计量,并且该化学计量受达因AAA3结构域的核苷酸状态调控。低亲和力状态要求Lis1还必须在新的保守位点上与动力蛋白结合,突变会破坏Lis1的体内功能。我们提出了一种新的Lis1调控动力蛋白的模型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号