当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sequential Release of Proteins from Structured Multishell Microcapsules
Biomacromolecules ( IF 5.5 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.biomac.7b00351 Ulyana Shimanovich 1, 2 , Thomas C. T. Michaels 1, 3 , Erwin De Genst 1 , Dijana Matak-Vinkovic 1 , Christopher M. Dobson 1 , Tuomas P. J. Knowles 1, 4
Biomacromolecules ( IF 5.5 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.biomac.7b00351 Ulyana Shimanovich 1, 2 , Thomas C. T. Michaels 1, 3 , Erwin De Genst 1 , Dijana Matak-Vinkovic 1 , Christopher M. Dobson 1 , Tuomas P. J. Knowles 1, 4
Affiliation
|
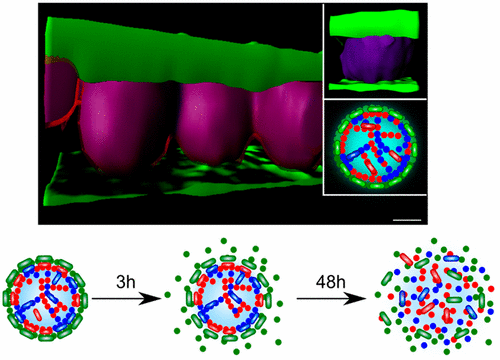
In nature, a wide range of functional materials is based on proteins. Increasing attention is also turning to the use of proteins as artificial biomaterials in the form of films, gels, particles, and fibrils that offer great potential for applications in areas ranging from molecular medicine to materials science. To date, however, most such applications have been limited to single component materials despite the fact that their natural analogues are composed of multiple types of proteins with a variety of functionalities that are coassembled in a highly organized manner on the micrometer scale, a process that is currently challenging to achieve in the laboratory. Here, we demonstrate the fabrication of multicomponent protein microcapsules where the different components are positioned in a controlled manner. We use molecular self-assembly to generate multicomponent structures on the nanometer scale and droplet microfluidics to bring together the different components on the micrometer scale. Using this approach, we synthesize a wide range of multiprotein microcapsules containing three well-characterized proteins: glucagon, insulin, and lysozyme. The localization of each protein component in multishell microcapsules has been detected by labeling protein molecules with different fluorophores, and the final three-dimensional microcapsule structure has been resolved by using confocal microscopy together with image analysis techniques. In addition, we show that these structures can be used to tailor the release of such functional proteins in a sequential manner. Moreover, our observations demonstrate that the protein release mechanism from multishell capsules is driven by the kinetic control of mass transport of the cargo and by the dissolution of the shells. The ability to generate artificial materials that incorporate a variety of different proteins with distinct functionalities increases the breadth of the potential applications of artificial protein-based materials and provides opportunities to design more refined functional protein delivery systems.
中文翻译:

从结构化多壳微胶囊中顺序释放蛋白质。
实际上,各种各样的功能材料都基于蛋白质。越来越多的注意力转向膜,凝胶,颗粒和原纤维形式的蛋白质作为人工生物材料的使用,这些分子在分子医学到材料科学等领域具有广阔的应用前景。然而,迄今为止,大多数这样的应用仅限于单组分材料,尽管它们的天然类似物由多种类型的蛋白质组成,这些蛋白质具有多种功能,这些蛋白质以微米级高度组织的方式组装在一起,这一过程目前在实验室中很难实现。在这里,我们演示了多组分蛋白质微囊的制造,其中不同组分以受控方式定位。我们使用分子自组装在纳米尺度上生成多组分结构,并使用微滴微滴将微米尺度上的不同组分聚集在一起。使用这种方法,我们合成了包含三种良好表征的蛋白质(胰高血糖素,胰岛素和溶菌酶)的多种蛋白质微囊。通过用不同的荧光团标记蛋白质分子,可以检测多壳微胶囊中每种蛋白质成分的定位,并通过使用共聚焦显微镜和图像分析技术来解析最终的三维微胶囊结构。另外,我们表明这些结构可用于以顺序方式定制此类功能性蛋白的释放。而且,我们的观察结果表明,从多壳胶囊释放蛋白质的机理是通过动力学控制货物的质量运输和通过壳的溶解来实现的。生成包含各种具有不同功能的不同蛋白质的人工材料的能力提高了基于人工蛋白质的材料的潜在应用范围,并为设计更完善的功能性蛋白质递送系统提供了机会。
更新日期:2017-09-07
中文翻译:

从结构化多壳微胶囊中顺序释放蛋白质。
实际上,各种各样的功能材料都基于蛋白质。越来越多的注意力转向膜,凝胶,颗粒和原纤维形式的蛋白质作为人工生物材料的使用,这些分子在分子医学到材料科学等领域具有广阔的应用前景。然而,迄今为止,大多数这样的应用仅限于单组分材料,尽管它们的天然类似物由多种类型的蛋白质组成,这些蛋白质具有多种功能,这些蛋白质以微米级高度组织的方式组装在一起,这一过程目前在实验室中很难实现。在这里,我们演示了多组分蛋白质微囊的制造,其中不同组分以受控方式定位。我们使用分子自组装在纳米尺度上生成多组分结构,并使用微滴微滴将微米尺度上的不同组分聚集在一起。使用这种方法,我们合成了包含三种良好表征的蛋白质(胰高血糖素,胰岛素和溶菌酶)的多种蛋白质微囊。通过用不同的荧光团标记蛋白质分子,可以检测多壳微胶囊中每种蛋白质成分的定位,并通过使用共聚焦显微镜和图像分析技术来解析最终的三维微胶囊结构。另外,我们表明这些结构可用于以顺序方式定制此类功能性蛋白的释放。而且,我们的观察结果表明,从多壳胶囊释放蛋白质的机理是通过动力学控制货物的质量运输和通过壳的溶解来实现的。生成包含各种具有不同功能的不同蛋白质的人工材料的能力提高了基于人工蛋白质的材料的潜在应用范围,并为设计更完善的功能性蛋白质递送系统提供了机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号