当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Dynamics of 15-Lipoxygenase-2 via Hydrogen–Deuterium Exchange
Biochemistry ( IF 2.9 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.biochem.7b00559 Kristin D. Droege 1 , Mary E. Keithly 2 , Charles R. Sanders 3 , Richard N. Armstrong 3 , Matthew K. Thompson 3
Biochemistry ( IF 2.9 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.biochem.7b00559 Kristin D. Droege 1 , Mary E. Keithly 2 , Charles R. Sanders 3 , Richard N. Armstrong 3 , Matthew K. Thompson 3
Affiliation
|
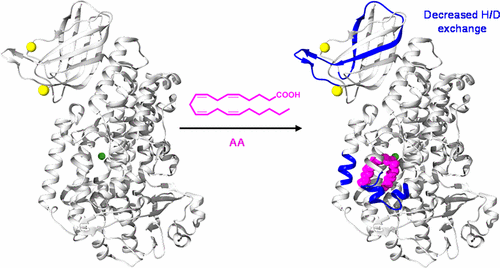
Eicosanoids are inflammatory signaling lipids that are biosynthesized in response to cellular injury or threat. They were originally thought to be pro-inflammatory molecules, but members of at least one subclass, the lipoxins, are able to resolve inflammation. One step in lipoxin synthesis is the oxygenation of arachidonic acid by 15-lipoxygenase (15-LOX). 15-LOX contains two domains: a Ca2+ binding PLAT domain and a catalytic domain. 15-LOX is a soluble cytosolic protein until binding of Ca2+ to the PLAT domain promotes translocation to the membrane surface. The role of 15-LOX structural dynamics in this translocation has remained unclear. We investigated the dynamics of 15-LOX isoform B (15-LOX-2) upon binding of Ca2+ and ligands, as well as upon membrane association using hydrogen–deuterium exchange mass spectrometry (HDX-MS). We used HDX-MS to probe the solvent accessibility and backbone flexibility of 15-LOX-2, revealing significant differences in deuterium incorporation between the PLAT and catalytic domains, with the PLAT domain demonstrating higher flexibility. Comparison of HDX for 15-LOX-2 in the presence and absence of Ca2+ indicates there are few differences in structural dynamics. Furthermore, our HDX results involving nanodisc-associated 15-LOX-2 suggest that significant structural and dynamic changes in 15-LOX-2 are not required for membrane association. Our results also show that a substrate lipid binding to the active site in the catalytic domain does induce changes in incorporation of deuterium into the PLAT domain. Overall, our results challenge the previous hypothesis that Ca2+ binding induces major structural changes in the PLAT domain and support the hypothesis that is interdomain communication in 15-LOX-2.
中文翻译:

15-Lipoxygenase-2通过氢-氘交换的结构动力学
类花生酸是响应细胞损伤或威胁而生物合成的炎症信号脂质。它们最初被认为是促炎分子,但是至少一种亚类脂蛋白的成员能够解决炎症。脂蛋白合成的一个步骤是通过15-脂氧合酶(15-LOX)进行花生四烯酸的氧合。15-LOX包含两个域:一个与Ca 2+结合的PLAT域和一个催化域。15-LOX是一种可溶性胞质蛋白,直到Ca 2+与PLAT结构域的结合促进了向膜表面的转运。15-LOX结构动力学在这种易位中的作用仍不清楚。我们研究了Ca 2+结合后15-LOX亚型B(15-LOX-2)的动力学和配体,以及使用氢-氘交换质谱(HDX-MS)进行膜结合时。我们使用HDX-MS探究了15-LOX-2的溶剂可及性和骨架柔性,揭示了PLAT和催化结构域之间氘掺入的显着差异,而PLAT结构域显示出更高的柔性。Ca 2+存在和不存在下15-LOX-2 HDX的比较表示结构动力学几乎没有差异。此外,我们涉及纳米光盘的15-LOX-2的HDX结果表明,膜缔合不需要15-LOX-2的显着结构和动态变化。我们的结果还表明,底物脂质结合至催化结构域中的活性位点的确会引起氘掺入PLAT结构域的变化。总体而言,我们的结果挑战了先前的假设,即Ca 2+结合会在PLAT域中诱导主要的结构变化,并支持15-LOX-2域间通讯的假设。
更新日期:2017-09-07
中文翻译:

15-Lipoxygenase-2通过氢-氘交换的结构动力学
类花生酸是响应细胞损伤或威胁而生物合成的炎症信号脂质。它们最初被认为是促炎分子,但是至少一种亚类脂蛋白的成员能够解决炎症。脂蛋白合成的一个步骤是通过15-脂氧合酶(15-LOX)进行花生四烯酸的氧合。15-LOX包含两个域:一个与Ca 2+结合的PLAT域和一个催化域。15-LOX是一种可溶性胞质蛋白,直到Ca 2+与PLAT结构域的结合促进了向膜表面的转运。15-LOX结构动力学在这种易位中的作用仍不清楚。我们研究了Ca 2+结合后15-LOX亚型B(15-LOX-2)的动力学和配体,以及使用氢-氘交换质谱(HDX-MS)进行膜结合时。我们使用HDX-MS探究了15-LOX-2的溶剂可及性和骨架柔性,揭示了PLAT和催化结构域之间氘掺入的显着差异,而PLAT结构域显示出更高的柔性。Ca 2+存在和不存在下15-LOX-2 HDX的比较表示结构动力学几乎没有差异。此外,我们涉及纳米光盘的15-LOX-2的HDX结果表明,膜缔合不需要15-LOX-2的显着结构和动态变化。我们的结果还表明,底物脂质结合至催化结构域中的活性位点的确会引起氘掺入PLAT结构域的变化。总体而言,我们的结果挑战了先前的假设,即Ca 2+结合会在PLAT域中诱导主要的结构变化,并支持15-LOX-2域间通讯的假设。











































 京公网安备 11010802027423号
京公网安备 11010802027423号