当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
pH-Dependent Binding of Chloride to a Marine Alkaline Phosphatase Affects the Catalysis, Active Site Stability, and Dimer Equilibrium
Biochemistry ( IF 2.9 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.biochem.7b00690 Jens G. Hjörleifsson 1 , Bjarni Ásgeirsson 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.biochem.7b00690 Jens G. Hjörleifsson 1 , Bjarni Ásgeirsson 1
Affiliation
|
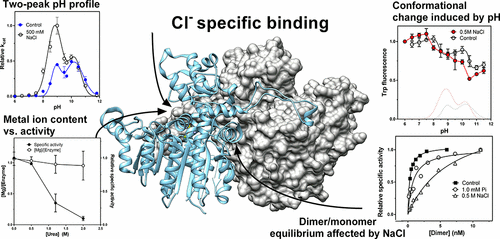
The effect of ionic strength on enzyme activity and stability varies considerably between enzymes. Ionic strength is known to affect the catalytic activity of some alkaline phosphatases (APs), such as Escherichia coli AP, but how ions affect APs is debated. Here, we studied the effect of various ions on a cold-adapted AP from Vibrio splendidus (VAP). Previously, we have found that the active form of VAP is extremely unstable at low ionic strengths. Here we show that NaCl increased the activity and stability of VAP and that the effect was pH-dependent in the range of pH 7–10. The activity profile as a function of pH formed two maxima, indicating a possible conformational change. Bringing the pH from the neutral to the alkaline range was accompanied by a large increase in both the Ki for inorganic phosphate (product inhibition) and the KM for p-nitrophenyl phosphate. The activity transitions observed as the pH was varied correlated with structural changes as monitored by tryptophan fluorescence. Thermal and urea-induced inactivation was shown to be accompanied by neither dissociation of the active site metal ions nor dimer dissociation. This would suggest that the inactivation involved subtle changes in active site conformation. Furthermore, the VAP dimer equilibrium was studied for the first time and shown to highly favor dimerization, which was dependent on pH and NaCl concentration. Taken together, the data support a model in which anions bind to some specific acceptor in the active site of VAP, resulting in great stabilization and catalytic rate enhancement, presumably through a different mechanism.
中文翻译:

pH依赖性氯化物与海洋碱性磷酸酶的结合影响催化,活性位点稳定性和二聚体平衡
离子强度对酶活性和稳定性的影响在酶之间差异很大。已知离子强度会影响某些碱性磷酸酶(AP)(例如大肠杆菌AP)的催化活性,但是人们还讨论了离子如何影响AP。在这里,我们研究了各种离子对Splendidus弧菌(VAP)的冷适应AP的影响。以前,我们发现活性形式的VAP在低离子强度下极其不稳定。在这里,我们表明NaCl可以提高VAP的活性和稳定性,并且在pH值为7-10的范围内,其影响取决于pH。随pH变化的活性曲线形成两个最大值,表明可能的构象变化。将pH从中性调至碱性时,两者的pH值均大幅增加。K i代表无机磷酸盐(抑制产物),K M代表p磷酸硝基苯酯。当pH变化时观察到的活性转变与通过色氨酸荧光监测的结构变化相关。热和尿素诱导的失活被证明既不伴有活性位点金属离子的解离,也不伴有二聚体的解离。这表明灭活涉及活性位点构象的细微变化。此外,首次对VAP二聚体平衡进行了研究,结果表明VAP二聚体高度依赖于pH值和NaCl浓度,因此非常有利于二聚化。两者合计,数据支持一个模型,在该模型中,阴离子与VAP活性位点上的某些特定受体结合,从而可能通过不同的机制实现了极大的稳定性和催化速率的提高。
更新日期:2017-09-07
中文翻译:

pH依赖性氯化物与海洋碱性磷酸酶的结合影响催化,活性位点稳定性和二聚体平衡
离子强度对酶活性和稳定性的影响在酶之间差异很大。已知离子强度会影响某些碱性磷酸酶(AP)(例如大肠杆菌AP)的催化活性,但是人们还讨论了离子如何影响AP。在这里,我们研究了各种离子对Splendidus弧菌(VAP)的冷适应AP的影响。以前,我们发现活性形式的VAP在低离子强度下极其不稳定。在这里,我们表明NaCl可以提高VAP的活性和稳定性,并且在pH值为7-10的范围内,其影响取决于pH。随pH变化的活性曲线形成两个最大值,表明可能的构象变化。将pH从中性调至碱性时,两者的pH值均大幅增加。K i代表无机磷酸盐(抑制产物),K M代表p磷酸硝基苯酯。当pH变化时观察到的活性转变与通过色氨酸荧光监测的结构变化相关。热和尿素诱导的失活被证明既不伴有活性位点金属离子的解离,也不伴有二聚体的解离。这表明灭活涉及活性位点构象的细微变化。此外,首次对VAP二聚体平衡进行了研究,结果表明VAP二聚体高度依赖于pH值和NaCl浓度,因此非常有利于二聚化。两者合计,数据支持一个模型,在该模型中,阴离子与VAP活性位点上的某些特定受体结合,从而可能通过不同的机制实现了极大的稳定性和催化速率的提高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号