当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functional Analysis of Cytochrome P450s Involved in Streptovaricin Biosynthesis and Generation of Anti-MRSA Analogues
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acschembio.7b00467 Yuanzhen Liu 1 , Xu Chen 1 , Zhengyuan Li 1 , Wei Xu 1 , Weixin Tao 1 , Jie Wu 2 , Jian Yang 2 , Zixin Deng 1, 3 , Yuhui Sun 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acschembio.7b00467 Yuanzhen Liu 1 , Xu Chen 1 , Zhengyuan Li 1 , Wei Xu 1 , Weixin Tao 1 , Jie Wu 2 , Jian Yang 2 , Zixin Deng 1, 3 , Yuhui Sun 1
Affiliation
|
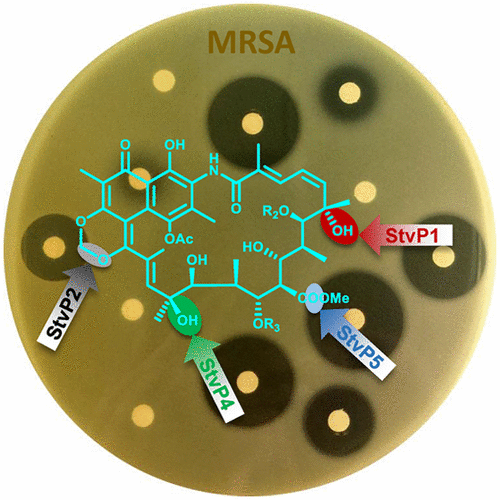
The streptovaricins, chemically related to the rifamycins, are highly effective antibacterial agents, particularly against mycobacteria. Herein, a bioassay-guided investigation of Streptomyces spectabilis CCTCC M2017417 has led to the characterization of streptovaricins as potent compounds against methicillin-resistant Staphylococcus aureus (MRSA). We identified the streptovaricin biosynthetic gene cluster from S. spectabilis CCTCC M2017417 based on genomic sequencing and bioinformatic analysis. Targeted in-frame deletion of five cytochrome P450 genes (stvP1–P5) resulted in the identification of four new streptovaricin analogues and revealed the functions of these genes as follows: stvP1, stvP4, and stvP5 are responsible for the hydroxylation of C-20, Me-24, and C-28, respectively. stvP2 is possibly involved in formation of the methylenedioxy bridge, and stvP3, a conserved gene found in the biosynthetic cluster for naphthalenic ansamycins, might be related to the formation of a naphthalene ring. Biochemical verification of the hydroxylase activity of StvP1, StvP4, and StvP5 was performed, and StvP1 showed unexpected biocatalytic specificity and promiscuity. More importantly, anti-MRSA studies of streptovaricins and derivatives revealed significant structure–activity relationships (SARs): The hydroxyl group at C-28 plays a vital role in antibacterial activity. The hydroxyl group at C-20 substantially enhances activity in the absence of the methoxycarbonyl side chain at C-24, which can increase the activity regardless of the presence of a hydroxyl group at C-20. The inner lactone ring between C-21 and C-24 shows a positive effect on activity. This work provides meaningful information on the SARs of streptovaricins and demonstrates the utility of the engineering of streptovaricins to yield novel anti-MRSA molecules.
中文翻译:

细胞色素P450参与链霉菌素生物合成和抗MRSA类似物的产生的功能分析。
与利福霉素化学相关的链霉菌素是高效的抗菌剂,尤其是针对分枝杆菌的抗菌剂。本文中,对链霉菌CCTCC M2017417进行生物测定指导的研究已表明,链霉菌素是有效的化合物,可抵抗耐甲氧西林的金黄色葡萄球菌(MRSA)。我们基于基因组测序和生物信息学分析,从壮观链球菌CCTCC M2017417中鉴定了链霉菌素生物合成基因簇。有针对性的框内删除五个细胞色素P450基因(stvP1 – P5)导致鉴定了四个新的链霉菌素类似物,并揭示了这些基因的功能,如下所示:stvP1,stvP4和stvP5分别负责C-20,Me-24和C-28的羟基化作用。stvP2可能参与亚甲二氧基桥的形成,而stvP3在萘合成的生物合成簇中发现的一个保守基因,可能与萘环的形成有关。生化验证StvP1,StvP4和StvP5的羟化酶活性,并且StvP1显示出乎意料的生物催化特异性和滥交。更重要的是,链霉菌素及其衍生物的抗MRSA研究显示出显着的构效关系(SAR):C-28处的羟基在抗菌活性中起着至关重要的作用。在C-24处不存在甲氧基羰基侧链的情况下,C-20处的羟基实质上增强了活性,这可以增加活性,而与C-20处是否存在羟基无关。C-21和C-24之间的内酯环对活性有积极作用。
更新日期:2017-09-07
中文翻译:

细胞色素P450参与链霉菌素生物合成和抗MRSA类似物的产生的功能分析。
与利福霉素化学相关的链霉菌素是高效的抗菌剂,尤其是针对分枝杆菌的抗菌剂。本文中,对链霉菌CCTCC M2017417进行生物测定指导的研究已表明,链霉菌素是有效的化合物,可抵抗耐甲氧西林的金黄色葡萄球菌(MRSA)。我们基于基因组测序和生物信息学分析,从壮观链球菌CCTCC M2017417中鉴定了链霉菌素生物合成基因簇。有针对性的框内删除五个细胞色素P450基因(stvP1 – P5)导致鉴定了四个新的链霉菌素类似物,并揭示了这些基因的功能,如下所示:stvP1,stvP4和stvP5分别负责C-20,Me-24和C-28的羟基化作用。stvP2可能参与亚甲二氧基桥的形成,而stvP3在萘合成的生物合成簇中发现的一个保守基因,可能与萘环的形成有关。生化验证StvP1,StvP4和StvP5的羟化酶活性,并且StvP1显示出乎意料的生物催化特异性和滥交。更重要的是,链霉菌素及其衍生物的抗MRSA研究显示出显着的构效关系(SAR):C-28处的羟基在抗菌活性中起着至关重要的作用。在C-24处不存在甲氧基羰基侧链的情况下,C-20处的羟基实质上增强了活性,这可以增加活性,而与C-20处是否存在羟基无关。C-21和C-24之间的内酯环对活性有积极作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号