当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Similarity Laws for the Lines of Ideal Free Energy and Chemical Potential in Supercritical Fluids
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.jpcb.7b07157 E. M. Apfelbaum 1 , V. S. Vorob’ev 1, 2
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.jpcb.7b07157 E. M. Apfelbaum 1 , V. S. Vorob’ev 1, 2
Affiliation
|
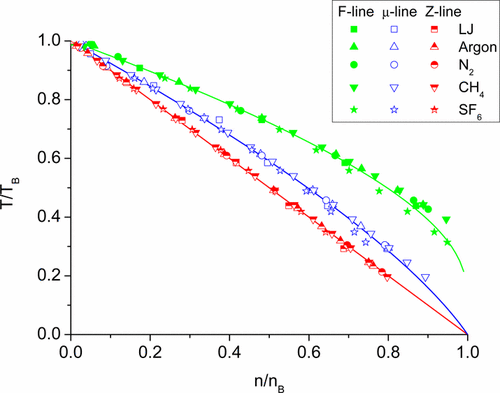
We have found the curves on the density–temperature plane, along which the values of free energy and chemical potential correspond to ideal gas quantities. At first, we have applied the van der Waals equation to construct them and to derive their equations. Then we have shown that the same lines for real substances (Ar, N2, CH4, SF6, H2, H2O) and for the model Lennard-Jones system constructed on the basis of the measurements data and calculations are well matched with the derived equations. The validity and deviations from the obtained similarity laws are discussed.
中文翻译:

超临界流体中理想自由能和化学势线的相似定律
我们在密度-温度平面上找到了曲线,自由能和化学势的值沿此曲线对应于理想气体量。首先,我们应用了van der Waals方程来构造它们并导出它们的方程。然后,我们表明对于实物(Ar,N 2,CH 4,SF 6,H 2,H 2 O)和基于测量数据和计算而构建的Lennard-Jones模型系统,相同的直线是很好的与导出的方程式匹配。讨论了所获得的相似性定律的有效性和偏差。
更新日期:2017-09-07
中文翻译:

超临界流体中理想自由能和化学势线的相似定律
我们在密度-温度平面上找到了曲线,自由能和化学势的值沿此曲线对应于理想气体量。首先,我们应用了van der Waals方程来构造它们并导出它们的方程。然后,我们表明对于实物(Ar,N 2,CH 4,SF 6,H 2,H 2 O)和基于测量数据和计算而构建的Lennard-Jones模型系统,相同的直线是很好的与导出的方程式匹配。讨论了所获得的相似性定律的有效性和偏差。











































 京公网安备 11010802027423号
京公网安备 11010802027423号