当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Using Peptide Aptamer Targeted Polymers as a Model Nanomedicine for Investigating Drug Distribution in Cancer Nanotheranostics
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00560 Yongmei Zhao 1 , Zachary H. Houston 1 , Joshua D. Simpson 1 , Liyu Chen 1 , Nicholas L. Fletcher 1 , Adrian V. Fuchs 1 , Idriss Blakey 1 , Kristofer J. Thurecht 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00560 Yongmei Zhao 1 , Zachary H. Houston 1 , Joshua D. Simpson 1 , Liyu Chen 1 , Nicholas L. Fletcher 1 , Adrian V. Fuchs 1 , Idriss Blakey 1 , Kristofer J. Thurecht 1
Affiliation
|
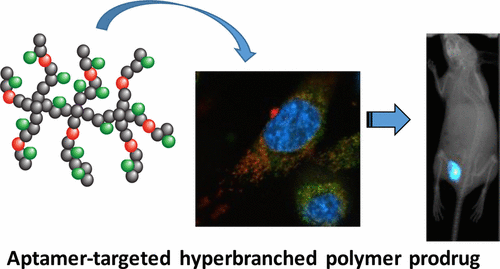
Theranostics is a strategy that combines multiple functions such as targeting, stimulus-responsive drug release, and diagnostic imaging into a single platform, often with the aim of developing personalized medicine.1,2 Based on this concept, several well-established hyperbranched polymeric theranostic nanoparticles were synthesized and characterized as model nanomedicines to investigate how their properties affect the distribution of loaded drugs at both the cell and whole animal levels. An 8-mer peptide aptamer was covalently bound to the periphery of the nanoparticles to achieve both targeting and potential chemosensitization functionality against heat shock protein 70 (Hsp70). Doxorubicin was also bound to the polymeric carrier as a model chemotherapeutic drug through a degradable hydrazone bond, enabling pH-controlled release under the mildly acid conditions that are found in the intracellular compartments of tumor cells. In order to track the nanoparticles, cyanine-5 (Cy5) was incorporated into the polymer as an optical imaging agent. In vitro cellular uptake was assessed for the hyperbranched polymer containing both doxorubicin (DOX) and Hsp70 targeted peptide aptamer in live MDA-MB-468 cells, and was found to be greater than that of either the untargeted, DOX-loaded polymer or polymer alone due to the specific affinity of the peptide aptamer for the breast cancer cells. This was also validated in vivo with the targeted polymers showing much higher accumulation within the tumor 48 h postinjection than the untargeted analogue. More detailed assessment of the nanomedicine distribution was achieved by directly following the polymeric carrier and the doxorubicin at both the in vitro cellular level via compartmental analysis of confocal images of live cells and in whole tumors ex vivo using confocal imaging to visualize the distribution of the drug in tumor tissue as a function of distance from blood vessels. Our results indicate that this polymeric carrier shows promise as a cancer theranostic, demonstrating active targeting to tumor cells with the capability for simultaneous drug release.
中文翻译:

使用肽适体靶向聚合物作为模型纳米药物研究癌症纳米治疗中的药物分布。
Theranostics是一种将多种功能(例如靶向,刺激性药物释放和诊断成像)组合到一个平台中的策略,通常旨在开发个性化药物。1,2基于此概念,几种成熟的超支化高分子治疗药物合成了纳米颗粒并将其表征为模型纳米药物,以研究纳米颗粒的特性如何影响细胞和整个动物水平上负载药物的分布。8聚肽适体共价结合到纳米粒子的外围,以实现针对热激蛋白70(Hsp70)的靶向和潜在的化学增敏功能。阿霉素还通过可降解的bond键作为模型化学治疗药物与聚合物载体结合,能够在肿瘤细胞胞内区室中存在的弱酸性条件下控制pH值的释放。为了跟踪纳米颗粒,将花菁5(Cy5)作为光学成像剂掺入到聚合物中。评估了活的MDA-MB-468细胞中同时含有阿霉素(DOX)和Hsp70靶向肽适体的超支链聚合物的体外细胞摄取,发现该摄取量大于未靶向的,载有DOX的聚合物或单独的聚合物由于肽适体对乳腺癌细胞的特异性亲和力。这也已在体内得到证实,目标聚合物在注射后48小时内在肿瘤内的蓄积要比未目标类似物高得多。通过对活细胞共聚焦图像和离体整个肿瘤的隔室分析,在体外细胞水平上直接跟踪聚合物载体和阿霉素在纳米细胞上的分布,可以对纳米药物的分布进行更详细的评估。使用共聚焦成像将药物在肿瘤组织中的分布可视化为距血管的距离的函数。我们的结果表明,这种聚合物载体具有治疗癌症的前景,表明它可以同时靶向释放具有同时释放药物能力的肿瘤细胞。
更新日期:2017-09-07
中文翻译:

使用肽适体靶向聚合物作为模型纳米药物研究癌症纳米治疗中的药物分布。
Theranostics是一种将多种功能(例如靶向,刺激性药物释放和诊断成像)组合到一个平台中的策略,通常旨在开发个性化药物。1,2基于此概念,几种成熟的超支化高分子治疗药物合成了纳米颗粒并将其表征为模型纳米药物,以研究纳米颗粒的特性如何影响细胞和整个动物水平上负载药物的分布。8聚肽适体共价结合到纳米粒子的外围,以实现针对热激蛋白70(Hsp70)的靶向和潜在的化学增敏功能。阿霉素还通过可降解的bond键作为模型化学治疗药物与聚合物载体结合,能够在肿瘤细胞胞内区室中存在的弱酸性条件下控制pH值的释放。为了跟踪纳米颗粒,将花菁5(Cy5)作为光学成像剂掺入到聚合物中。评估了活的MDA-MB-468细胞中同时含有阿霉素(DOX)和Hsp70靶向肽适体的超支链聚合物的体外细胞摄取,发现该摄取量大于未靶向的,载有DOX的聚合物或单独的聚合物由于肽适体对乳腺癌细胞的特异性亲和力。这也已在体内得到证实,目标聚合物在注射后48小时内在肿瘤内的蓄积要比未目标类似物高得多。通过对活细胞共聚焦图像和离体整个肿瘤的隔室分析,在体外细胞水平上直接跟踪聚合物载体和阿霉素在纳米细胞上的分布,可以对纳米药物的分布进行更详细的评估。使用共聚焦成像将药物在肿瘤组织中的分布可视化为距血管的距离的函数。我们的结果表明,这种聚合物载体具有治疗癌症的前景,表明它可以同时靶向释放具有同时释放药物能力的肿瘤细胞。











































 京公网安备 11010802027423号
京公网安备 11010802027423号