当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Para-Fluoro Postpolymerization Chemistry of Poly(pentafluorobenzyl methacrylate): Modification with Amines, Thiols, and Carbonylthiolates
Macromolecules ( IF 5.1 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.macromol.7b01603 Janina-Miriam Noy 1 , Ann-Katrin Friedrich 1 , Kyle Batten 2 , Mathamsanqa N. Bhebhe 2 , Nicolas Busatto 3 , Rhiannon R. Batchelor 1 , Ariella Kristanti 1 , Yiwen Pei 1, 2, 4 , Peter J. Roth 1, 2, 3
Macromolecules ( IF 5.1 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.macromol.7b01603 Janina-Miriam Noy 1 , Ann-Katrin Friedrich 1 , Kyle Batten 2 , Mathamsanqa N. Bhebhe 2 , Nicolas Busatto 3 , Rhiannon R. Batchelor 1 , Ariella Kristanti 1 , Yiwen Pei 1, 2, 4 , Peter J. Roth 1, 2, 3
Affiliation
|
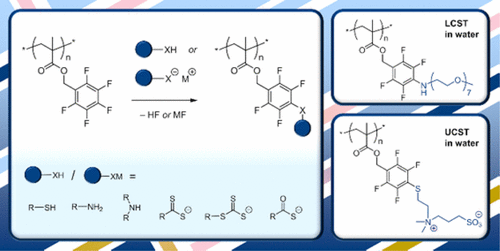
A methacrylic polymer undergoing highly efficient para-fluoro substitution reactions is presented. A series of well-defined poly(2,3,4,5,6-pentafluorobenzyl methacrylate) (pPFBMA) homopolymers with degrees of polymerization from 28 to 132 and Đ ≤ 1.29 was prepared by the RAFT process. pPFBMA samples were atactic (with triad tacticity apparent in 1H and 19F NMR spectra) and soluble in most organic solvents. pPFBMA reacted quantitatively through para-fluoro substitution with a range of thiols (typically 1.1 equiv of thiol, base, RT, <1 h) in the absence of any observed side reactions. Para-fluoro substitution with different (thio)carbonylthio reagents was possible and allowed for subsequent one-pot cleavage of dithioester pendent groups with concurrent thia-Michael side group modification. Reactions with aliphatic amines (typically 2.5 equiv of amine, 50–60 °C, overnight) resulted in complete substitution of the para-fluorides without any observed ester cleavage reactions. However, for primary amines, H2NR, double substitution reactions yielding tertiary (−C6F4)2NR amine bridges were observed, which were absent with secondary amine reagents. No reactions were found for attempted modifications of pPFBMA with bromide, iodide, methanethiosulfonate, or thiourea, indicating a highly selective reactivity toward nucleophiles. The versatility of this reactive platform is demonstrated through the synthesis of a pH-responsive polymer and novel thermoresponsive polymers: an oligo(ethylene glycol)-functional species with an LCST in water and two zwitterionic polymers with UCSTs in water and aqueous salt solution (NaCl concentration up to 178 mM).
中文翻译:

聚(五氟甲基丙烯酸甲酯)的对氟后聚合化学:胺,硫醇和羰基硫醇盐的改性
提出了进行高效对氟取代反应的甲基丙烯酸聚合物。与聚合度为28至132和一系列的良好定义的聚(2,3,4,5,6-五氟苄基甲基丙烯酸酯)(pPFBMA)均聚物Đ ≤1.29通过RAFT方法制备。pPFBMA样品是无规立构的(在1 H和19 F NMR光谱中具有明显的三单元组立构规整度),可溶于大多数有机溶剂。在没有观察到任何副反应的情况下,pPFBMA通过对氟取代与一系列硫醇(通常为1.1当量的硫醇,碱,RT,<1 h)进行定量反应。帕拉用不同的(硫代)羰基硫基试剂进行氟取代是可能的,并允许随后的一硫键对二硫酯侧基进行一锅裂解,同时进行thia-Michael侧基修饰。与脂肪胺的反应(通常为2.5当量的胺,在50–60°C下过夜)导致对氟化物的完全取代,而没有观察到任何酯裂解反应。但是,对于伯胺H 2 NR,双取代反应会生成叔(-C 6 F 4)2观察到了仲胺试剂不存在的NR胺桥。未发现尝试用溴化物,碘化物,甲硫代磺酸盐或硫脲修饰pPFBMA的反应,表明对亲核试剂具有高度选择性。该反应平台的多功能性通过合成pH响应性聚合物和新型热响应性聚合物得到证明:一种在水中具有LCST的低聚(乙二醇)功能物种,以及在水和盐水溶液(NaCl)中具有UCST的两种两性离子聚合物浓度高达178 mM)。
更新日期:2017-09-07
中文翻译:

聚(五氟甲基丙烯酸甲酯)的对氟后聚合化学:胺,硫醇和羰基硫醇盐的改性
提出了进行高效对氟取代反应的甲基丙烯酸聚合物。与聚合度为28至132和一系列的良好定义的聚(2,3,4,5,6-五氟苄基甲基丙烯酸酯)(pPFBMA)均聚物Đ ≤1.29通过RAFT方法制备。pPFBMA样品是无规立构的(在1 H和19 F NMR光谱中具有明显的三单元组立构规整度),可溶于大多数有机溶剂。在没有观察到任何副反应的情况下,pPFBMA通过对氟取代与一系列硫醇(通常为1.1当量的硫醇,碱,RT,<1 h)进行定量反应。帕拉用不同的(硫代)羰基硫基试剂进行氟取代是可能的,并允许随后的一硫键对二硫酯侧基进行一锅裂解,同时进行thia-Michael侧基修饰。与脂肪胺的反应(通常为2.5当量的胺,在50–60°C下过夜)导致对氟化物的完全取代,而没有观察到任何酯裂解反应。但是,对于伯胺H 2 NR,双取代反应会生成叔(-C 6 F 4)2观察到了仲胺试剂不存在的NR胺桥。未发现尝试用溴化物,碘化物,甲硫代磺酸盐或硫脲修饰pPFBMA的反应,表明对亲核试剂具有高度选择性。该反应平台的多功能性通过合成pH响应性聚合物和新型热响应性聚合物得到证明:一种在水中具有LCST的低聚(乙二醇)功能物种,以及在水和盐水溶液(NaCl)中具有UCST的两种两性离子聚合物浓度高达178 mM)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号