当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stabilising Ni catalysts for the dehydration–decarboxylation–hydrogenation of citric acid to methylsuccinic acid
Green Chemistry ( IF 9.3 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1039/c7gc01773a Jasper Verduyckt 1, 2, 3, 4, 5 , Anton Geers 1, 2, 3, 4, 5 , Birgit Claes 1, 2, 3, 4, 5 , Samuel Eyley 3, 6, 7, 8, 9 , Cédric Van Goethem 1, 2, 3, 4, 5 , Ivo Stassen 1, 2, 3, 4, 5 , Simon Smolders 1, 2, 3, 4, 5 , Rob Ameloot 1, 2, 3, 4, 5 , Ivo Vankelecom 1, 2, 3, 4, 5 , Wim Thielemans 3, 6, 7, 8, 9 , Dirk E. De Vos 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1039/c7gc01773a Jasper Verduyckt 1, 2, 3, 4, 5 , Anton Geers 1, 2, 3, 4, 5 , Birgit Claes 1, 2, 3, 4, 5 , Samuel Eyley 3, 6, 7, 8, 9 , Cédric Van Goethem 1, 2, 3, 4, 5 , Ivo Stassen 1, 2, 3, 4, 5 , Simon Smolders 1, 2, 3, 4, 5 , Rob Ameloot 1, 2, 3, 4, 5 , Ivo Vankelecom 1, 2, 3, 4, 5 , Wim Thielemans 3, 6, 7, 8, 9 , Dirk E. De Vos 1, 2, 3, 4, 5
Affiliation
|
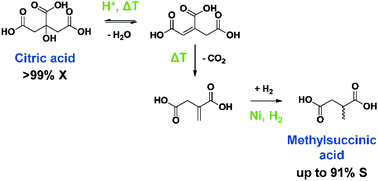
A new reaction sequence of dehydration–decarboxylation–hydrogenation to transform citric acid into methylsuccinic acid has recently been developed using Pd as a noble metal catalyst in water. In this work Ni catalysts were investigated as low cost, non-noble metal alternatives. Several home-made and commercial catalysts were screened for this reaction. Citric acid was very reactive and full conversions were readily obtained in all cases. However, the selectivity to methylsuccinic acid was initially low, since typical Ni catalysts were not stable and therefore not able to hydrogenate the formed C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C double bonds. Due to the lower hydrogenation activity of Ni compared to Pd, new side products appeared. Particularly, hydration of the C
C double bonds. Due to the lower hydrogenation activity of Ni compared to Pd, new side products appeared. Particularly, hydration of the C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C double bonds made the reaction network more complex in this case. Fortunately, the formation of all hydration products – even the rather stable lactone, β-carboxy-γ-butyrolactone – was eventually shown to be completely reversible. Three routes were then studied to stabilise Ni catalysts and to enable the Ni catalyzed conversion of citric acid to methylsuccinic acid; partial neutralisation of the acid reactant, adding Fe to Ni/ZrO2 or to the reaction mixture and coating Ni particles with carbon, all proved to stabilise Ni and all resulted in high to very high methylsuccinic acid yields. Furthermore, the role of Fe was unravelled by performing reference reactions with different Fe compounds and by in depth characterisation of the NiFe/ZrO2 catalyst. Finally, the reaction conditions were optimised using the carbon-coated Ni nanoparticles and kinetic profiles were recorded to confirm the extended reaction network.
C double bonds made the reaction network more complex in this case. Fortunately, the formation of all hydration products – even the rather stable lactone, β-carboxy-γ-butyrolactone – was eventually shown to be completely reversible. Three routes were then studied to stabilise Ni catalysts and to enable the Ni catalyzed conversion of citric acid to methylsuccinic acid; partial neutralisation of the acid reactant, adding Fe to Ni/ZrO2 or to the reaction mixture and coating Ni particles with carbon, all proved to stabilise Ni and all resulted in high to very high methylsuccinic acid yields. Furthermore, the role of Fe was unravelled by performing reference reactions with different Fe compounds and by in depth characterisation of the NiFe/ZrO2 catalyst. Finally, the reaction conditions were optimised using the carbon-coated Ni nanoparticles and kinetic profiles were recorded to confirm the extended reaction network.
中文翻译:

用于柠檬酸脱水-脱羧-加氢为甲基琥珀酸的稳定化镍催化剂
最近,使用钯作为水中的贵金属催化剂,开发了一种脱水-脱羧-加氢将柠檬酸转化为甲基琥珀酸的新反应顺序。在这项工作中,作为低成本,非贵金属的替代品,对镍催化剂进行了研究。筛选了几种家用和商用催化剂用于该反应。柠檬酸是非常活泼的,在所有情况下都容易获得完全转化。然而,由于典型的Ni催化剂不稳定并且因此不能氢化所形成的C![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) C双键,因此对甲基琥珀酸的选择性最初较低。由于Ni的氢化活性低于Pd,因此出现了新的副产物。特别是C的水合
C双键,因此对甲基琥珀酸的选择性最初较低。由于Ni的氢化活性低于Pd,因此出现了新的副产物。特别是C的水合![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) 在这种情况下,C双键使反应网络更复杂。幸运的是,所有水合产物的形成,甚至是相当稳定的内酯,β-羧基-γ-丁内酯,最终都被证明是完全可逆的。然后研究了三种途径来稳定镍催化剂并使镍催化的柠檬酸转化为甲基琥珀酸。对酸反应物进行部分中和,将铁添加到Ni / ZrO 2或反应混合物中,并用碳覆盖Ni颗粒,都证明可以稳定Ni,并且都可以产生很高或非常高的甲基琥珀酸收率。此外,通过与不同的Fe化合物进行参考反应并通过深度表征NiFe / ZrO 2来阐明Fe的作用催化剂。最后,使用碳包覆的镍纳米粒子优化了反应条件,并记录了动力学曲线以确认扩展的反应网络。
在这种情况下,C双键使反应网络更复杂。幸运的是,所有水合产物的形成,甚至是相当稳定的内酯,β-羧基-γ-丁内酯,最终都被证明是完全可逆的。然后研究了三种途径来稳定镍催化剂并使镍催化的柠檬酸转化为甲基琥珀酸。对酸反应物进行部分中和,将铁添加到Ni / ZrO 2或反应混合物中,并用碳覆盖Ni颗粒,都证明可以稳定Ni,并且都可以产生很高或非常高的甲基琥珀酸收率。此外,通过与不同的Fe化合物进行参考反应并通过深度表征NiFe / ZrO 2来阐明Fe的作用催化剂。最后,使用碳包覆的镍纳米粒子优化了反应条件,并记录了动力学曲线以确认扩展的反应网络。
更新日期:2017-09-07
![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C double bonds. Due to the lower hydrogenation activity of Ni compared to Pd, new side products appeared. Particularly, hydration of the C
C double bonds. Due to the lower hydrogenation activity of Ni compared to Pd, new side products appeared. Particularly, hydration of the C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C double bonds made the reaction network more complex in this case. Fortunately, the formation of all hydration products – even the rather stable lactone, β-carboxy-γ-butyrolactone – was eventually shown to be completely reversible. Three routes were then studied to stabilise Ni catalysts and to enable the Ni catalyzed conversion of citric acid to methylsuccinic acid; partial neutralisation of the acid reactant, adding Fe to Ni/ZrO2 or to the reaction mixture and coating Ni particles with carbon, all proved to stabilise Ni and all resulted in high to very high methylsuccinic acid yields. Furthermore, the role of Fe was unravelled by performing reference reactions with different Fe compounds and by in depth characterisation of the NiFe/ZrO2 catalyst. Finally, the reaction conditions were optimised using the carbon-coated Ni nanoparticles and kinetic profiles were recorded to confirm the extended reaction network.
C double bonds made the reaction network more complex in this case. Fortunately, the formation of all hydration products – even the rather stable lactone, β-carboxy-γ-butyrolactone – was eventually shown to be completely reversible. Three routes were then studied to stabilise Ni catalysts and to enable the Ni catalyzed conversion of citric acid to methylsuccinic acid; partial neutralisation of the acid reactant, adding Fe to Ni/ZrO2 or to the reaction mixture and coating Ni particles with carbon, all proved to stabilise Ni and all resulted in high to very high methylsuccinic acid yields. Furthermore, the role of Fe was unravelled by performing reference reactions with different Fe compounds and by in depth characterisation of the NiFe/ZrO2 catalyst. Finally, the reaction conditions were optimised using the carbon-coated Ni nanoparticles and kinetic profiles were recorded to confirm the extended reaction network.
中文翻译:

用于柠檬酸脱水-脱羧-加氢为甲基琥珀酸的稳定化镍催化剂
最近,使用钯作为水中的贵金属催化剂,开发了一种脱水-脱羧-加氢将柠檬酸转化为甲基琥珀酸的新反应顺序。在这项工作中,作为低成本,非贵金属的替代品,对镍催化剂进行了研究。筛选了几种家用和商用催化剂用于该反应。柠檬酸是非常活泼的,在所有情况下都容易获得完全转化。然而,由于典型的Ni催化剂不稳定并且因此不能氢化所形成的C
![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) C双键,因此对甲基琥珀酸的选择性最初较低。由于Ni的氢化活性低于Pd,因此出现了新的副产物。特别是C的水合
C双键,因此对甲基琥珀酸的选择性最初较低。由于Ni的氢化活性低于Pd,因此出现了新的副产物。特别是C的水合![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) 在这种情况下,C双键使反应网络更复杂。幸运的是,所有水合产物的形成,甚至是相当稳定的内酯,β-羧基-γ-丁内酯,最终都被证明是完全可逆的。然后研究了三种途径来稳定镍催化剂并使镍催化的柠檬酸转化为甲基琥珀酸。对酸反应物进行部分中和,将铁添加到Ni / ZrO 2或反应混合物中,并用碳覆盖Ni颗粒,都证明可以稳定Ni,并且都可以产生很高或非常高的甲基琥珀酸收率。此外,通过与不同的Fe化合物进行参考反应并通过深度表征NiFe / ZrO 2来阐明Fe的作用催化剂。最后,使用碳包覆的镍纳米粒子优化了反应条件,并记录了动力学曲线以确认扩展的反应网络。
在这种情况下,C双键使反应网络更复杂。幸运的是,所有水合产物的形成,甚至是相当稳定的内酯,β-羧基-γ-丁内酯,最终都被证明是完全可逆的。然后研究了三种途径来稳定镍催化剂并使镍催化的柠檬酸转化为甲基琥珀酸。对酸反应物进行部分中和,将铁添加到Ni / ZrO 2或反应混合物中,并用碳覆盖Ni颗粒,都证明可以稳定Ni,并且都可以产生很高或非常高的甲基琥珀酸收率。此外,通过与不同的Fe化合物进行参考反应并通过深度表征NiFe / ZrO 2来阐明Fe的作用催化剂。最后,使用碳包覆的镍纳米粒子优化了反应条件,并记录了动力学曲线以确认扩展的反应网络。











































 京公网安备 11010802027423号
京公网安备 11010802027423号