当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploiting Overlapping Advantages of In Vitro and In Cellulo Selection Systems to Isolate a Novel High-Affinity cJun Antagonist
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acschembio.7b00693 Daniel Baxter 1, 2 , Christopher G Ullman 2 , Laura Frigotto 2 , Jody M Mason 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acschembio.7b00693 Daniel Baxter 1, 2 , Christopher G Ullman 2 , Laura Frigotto 2 , Jody M Mason 1
Affiliation
|
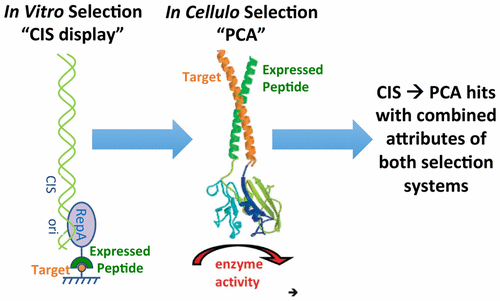
We have combined two peptide library-screening systems, exploiting the benefits offered by both to select novel antagonistic agents of cJun. CIS display is an in vitro cell-free system that allows very large libraries (≤1014) to be interrogated. However, affinity-based screening conditions can poorly reflect those relevant to therapeutic application, particularly for difficult intracellular targets, and can lead to false positives. In contrast, an in cellulo screening system such as the Protein-fragment Complementation Assay (PCA) selects peptides with high target affinity while additionally profiling for target specificity, protease resistance, solubility, and lack of toxicity in a more relevant context. A disadvantage is the necessity to transform cells, limiting library sizes that can be screened to ≤106. However, by combining both cell-free and cell-based systems, we isolated a peptide (CPW) from a ∼1010 member library, which forms a highly stable interaction with cJun (Tm = 63 °C, Kd = 750 nM, ΔG = −8.2 kcal/mol) using the oncogenic transcriptional regulator Activator Protein-1 (AP-1) as our exemplar target. In contrast, CIS display alone selected a peptide with low affinity for cJun (Tm = 34 °C, Kd = 25 μM, ΔG = −6.2 kcal/mol), highlighting the benefit of CIS → PCA. Furthermore, increased library size with CIS → PCA vs PCA alone allows the freedom to introduce noncanonical options, such as interfacial aromatics, and solvent exposed options that may allow the molecule to explore alternative structures and interact with greater affinity and efficacy with the target. CIS → PCA therefore offers significant potential as a peptide-library screening platform by synergistically combining the relative attributes of both assays to generate therapeutically interesting compounds that may otherwise not be identified.
中文翻译:

利用体外和细胞内选择系统的重叠优势来分离一种新型的高亲和力 cJun 拮抗剂
我们结合了两个肽库筛选系统,利用两者提供的优势来选择 cJun 的新型拮抗剂。CIS 展示是一种体外无细胞系统,允许对非常大的库 (≤10 14 ) 进行询问。然而,基于亲和力的筛选条件不能很好地反映与治疗应用相关的条件,特别是对于困难的细胞内靶标,并且可能导致假阳性。相比之下,在蜂窝筛选系统如蛋白质片段互补分析 (PCA) 选择具有高靶标亲和力的肽,同时在更相关的环境中另外分析靶标特异性、蛋白酶抗性、溶解性和无毒性。一个缺点是必须转换单元格,将可筛选的库大小限制为≤10 6。然而,通过结合无细胞和基于细胞的系统,我们从~ 10 10 个成员库中分离出一个肽(CPW),它与 cJun 形成高度稳定的相互作用(T m = 63 °C,K d = 750 nM , Δ G= -8.2 kcal/mol) 使用致癌转录调节因子激活蛋白 1 (AP-1) 作为我们的示例靶标。相比之下,单独的 CIS 展示选择了对 cJun 亲和力低的肽 ( T m = 34 °C, K d = 25 μM, Δ G= -6.2 kcal/mol),突出了 CIS → PCA 的好处。此外,使用 CIS → PCA 与单独的 PCA 相比,增加的库大小允许自由引入非规范选项,例如界面芳烃和溶剂暴露选项,这些选项可能允许分子探索替代结构并以更大的亲和力和与靶标相互作用。因此,CIS → PCA 通过协同结合两种检测方法的相关属性来生成可能无法识别的具有治疗意义的化合物,从而提供了作为肽库筛选平台的巨大潜力。
更新日期:2017-09-07
中文翻译:

利用体外和细胞内选择系统的重叠优势来分离一种新型的高亲和力 cJun 拮抗剂
我们结合了两个肽库筛选系统,利用两者提供的优势来选择 cJun 的新型拮抗剂。CIS 展示是一种体外无细胞系统,允许对非常大的库 (≤10 14 ) 进行询问。然而,基于亲和力的筛选条件不能很好地反映与治疗应用相关的条件,特别是对于困难的细胞内靶标,并且可能导致假阳性。相比之下,在蜂窝筛选系统如蛋白质片段互补分析 (PCA) 选择具有高靶标亲和力的肽,同时在更相关的环境中另外分析靶标特异性、蛋白酶抗性、溶解性和无毒性。一个缺点是必须转换单元格,将可筛选的库大小限制为≤10 6。然而,通过结合无细胞和基于细胞的系统,我们从~ 10 10 个成员库中分离出一个肽(CPW),它与 cJun 形成高度稳定的相互作用(T m = 63 °C,K d = 750 nM , Δ G= -8.2 kcal/mol) 使用致癌转录调节因子激活蛋白 1 (AP-1) 作为我们的示例靶标。相比之下,单独的 CIS 展示选择了对 cJun 亲和力低的肽 ( T m = 34 °C, K d = 25 μM, Δ G= -6.2 kcal/mol),突出了 CIS → PCA 的好处。此外,使用 CIS → PCA 与单独的 PCA 相比,增加的库大小允许自由引入非规范选项,例如界面芳烃和溶剂暴露选项,这些选项可能允许分子探索替代结构并以更大的亲和力和与靶标相互作用。因此,CIS → PCA 通过协同结合两种检测方法的相关属性来生成可能无法识别的具有治疗意义的化合物,从而提供了作为肽库筛选平台的巨大潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号