当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A New Method To Identify Physically Stable Concentration of Amorphous Solid Dispersions (I): Case of Flutamide + Kollidon VA64
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00382 K. Chmiel 1, 2 , J. Knapik-Kowalczuk 1, 2 , K. Jurkiewicz 1, 2 , W. Sawicki 3 , R. Jachowicz 4 , M. Paluch 1, 2
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00382 K. Chmiel 1, 2 , J. Knapik-Kowalczuk 1, 2 , K. Jurkiewicz 1, 2 , W. Sawicki 3 , R. Jachowicz 4 , M. Paluch 1, 2
Affiliation
|
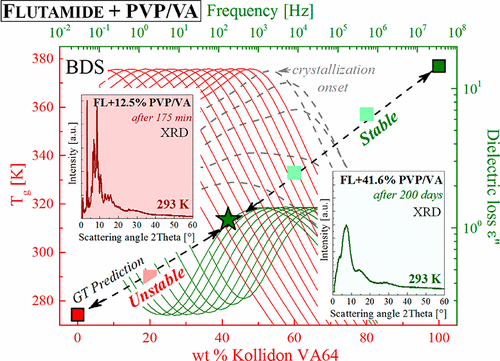
In this paper, a novel approach to determine stable concentration in API-polymer systems is presented. As a model, binary amorphous mixtures flutamide (FL) drug with a copolymer Kollidon VA64 (PVP/VA) have been used. It is worthwhile to note that finding an effective method to achieve this goal is a matter of great importance because physical stability of the amorphous pharmaceuticals is the key issue that is investigated worldwide. Due to the fact that molecular dynamics was found to be the crucial factor affecting physical stability of disordered pharmaceuticals, we examined it for both neat FL and its PVP/VA mixtures by means of broadband dielectric spectroscopy (BDS). Thorough investigation of the impact of polymeric additive on the molecular mobility of disordered FL reveals unusual, previously unreported behavior. Namely, simultaneously with the beginning of the recrystallization process, we observe some transformation from unstable supersaturated concentration of investigated mixture to the different, unknown concentration of FL-PVP/VA. Observed, during BDS experiment, transformation enables us to determine the limiting, highly physically stable concentration of FL in PVP/VA polymer (saturated solution), which is equivalent to FL + 41% wt. of PVP/VA. The described high physical stability of this unveiled system has been confirmed by means of long-term XRD measurements. According to our knowledge, this is the first time when such a behavior has been observed by means of BDS.
中文翻译:

一种确定非晶态固体分散体物理稳定浓度的新方法(I):氟他胺+ Kollidon VA64的案例
在本文中,提出了一种确定API聚合物系统中稳定浓度的新颖方法。作为模型,已使用二元无定形混合物氟他胺(FL)药物与共聚物Kollidon VA64(PVP / VA)。值得指出的是,找到一种有效的方法来实现这一目标非常重要,因为无定形药物的物理稳定性是全世界研究的关键问题。由于发现分子动力学是影响无序药物物理稳定性的关键因素,我们通过宽带介电谱(BDS)对纯净FL及其PVP / VA混合物进行了检查。彻底研究聚合物添加剂对无序FL分子迁移的影响,发现了以前未报道的异常行为。即 在重结晶过程开始的同时,我们观察到了从不稳定的过饱和浓度的研究混合物到不同未知浓度的FL-PVP / VA的一些转变。观察到,在BDS实验期间,转化使我们能够确定PVP / VA聚合物(饱和溶液)中FL的极限,高度物理稳定的浓度,相当于FL + 41%wt。PVP / VA。通过长期XRD测量,已证实了该公开系统所描述的高物理稳定性。据我们所知,这是第一次通过BDS观察到这种行为。FL-PVP / VA的浓度未知。观察到,在BDS实验期间,转化使我们能够确定PVP / VA聚合物(饱和溶液)中FL的极限,高度物理稳定的浓度,相当于FL + 41%wt。PVP / VA。通过长期XRD测量,已证实了该公开系统所描述的高物理稳定性。据我们所知,这是第一次通过BDS观察到这种行为。FL-PVP / VA的浓度未知。观察到,在BDS实验期间,转化使我们能够确定PVP / VA聚合物(饱和溶液)中FL的极限,高度物理稳定的浓度,相当于FL + 41%wt。PVP / VA。通过长期XRD测量,已证实了该公开系统所描述的高物理稳定性。据我们所知,这是第一次通过BDS观察到这种行为。
更新日期:2017-09-07
中文翻译:

一种确定非晶态固体分散体物理稳定浓度的新方法(I):氟他胺+ Kollidon VA64的案例
在本文中,提出了一种确定API聚合物系统中稳定浓度的新颖方法。作为模型,已使用二元无定形混合物氟他胺(FL)药物与共聚物Kollidon VA64(PVP / VA)。值得指出的是,找到一种有效的方法来实现这一目标非常重要,因为无定形药物的物理稳定性是全世界研究的关键问题。由于发现分子动力学是影响无序药物物理稳定性的关键因素,我们通过宽带介电谱(BDS)对纯净FL及其PVP / VA混合物进行了检查。彻底研究聚合物添加剂对无序FL分子迁移的影响,发现了以前未报道的异常行为。即 在重结晶过程开始的同时,我们观察到了从不稳定的过饱和浓度的研究混合物到不同未知浓度的FL-PVP / VA的一些转变。观察到,在BDS实验期间,转化使我们能够确定PVP / VA聚合物(饱和溶液)中FL的极限,高度物理稳定的浓度,相当于FL + 41%wt。PVP / VA。通过长期XRD测量,已证实了该公开系统所描述的高物理稳定性。据我们所知,这是第一次通过BDS观察到这种行为。FL-PVP / VA的浓度未知。观察到,在BDS实验期间,转化使我们能够确定PVP / VA聚合物(饱和溶液)中FL的极限,高度物理稳定的浓度,相当于FL + 41%wt。PVP / VA。通过长期XRD测量,已证实了该公开系统所描述的高物理稳定性。据我们所知,这是第一次通过BDS观察到这种行为。FL-PVP / VA的浓度未知。观察到,在BDS实验期间,转化使我们能够确定PVP / VA聚合物(饱和溶液)中FL的极限,高度物理稳定的浓度,相当于FL + 41%wt。PVP / VA。通过长期XRD测量,已证实了该公开系统所描述的高物理稳定性。据我们所知,这是第一次通过BDS观察到这种行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号